A1.1 -- Water
1/57
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
condensation reaction
(JOINS)
water formed as a product when two molecules join together.
hydrolysis reaction
(SPLITS)
water reacts with a chemical to break it into smaller molecules.
the three constituents / parts of an atom
proton
neutron
electron
atoms are neutral when…
they have an equal number of protons and electrons.
atoms become charged when….
there is either a deficit or surplus of electrons.
atom
the smallest particle of a chemical element that can exist
element
matter that cannot be decomposed into something simpler
*all atoms of an element have the same number of protons
molecule
atoms that are joined together by a molecular bond
compound
a molecule made up of two or more different atoms
chemical bond
an attraction between atoms/ions/molecules that enables the formation of chemical compounds
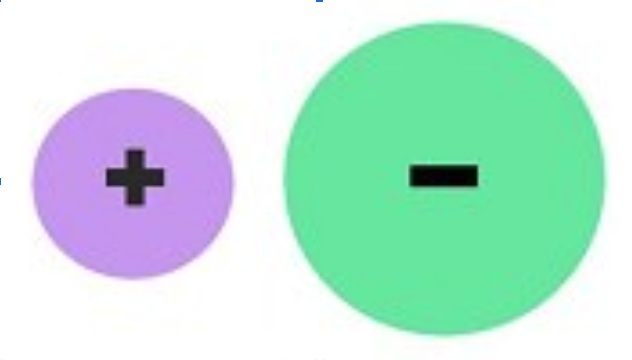
ionic bond
involves the transfer of electrons
cation = positive charge
anion = negative charge
covalent bond
involves the sharing of electrons
types of chemical bonds
non-polar covalent bond
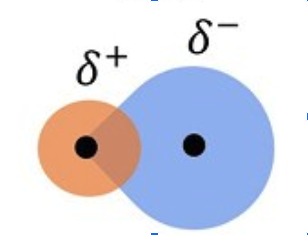
polar covalent bond
ionic bond
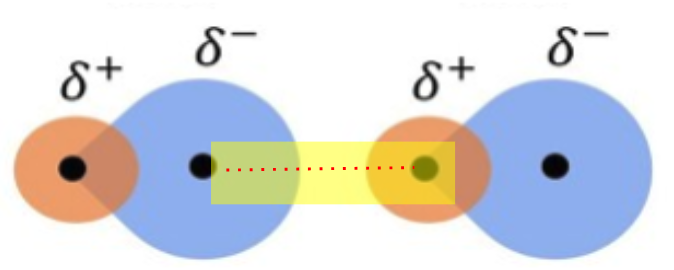
hydrogen bond
non-polar covalent bond
electrons are shared equally
polar covalent bond
electrons are shared unequally
ionic bond (within a molecule)
attraction between a positively charged ion (cation) and a negatively charged ion (anion)
hydrogen bond (between different molecules)
attraction between 𝛿+ and 𝛿− regions of two different polar molecules
*intermolecular force, not an actual bond
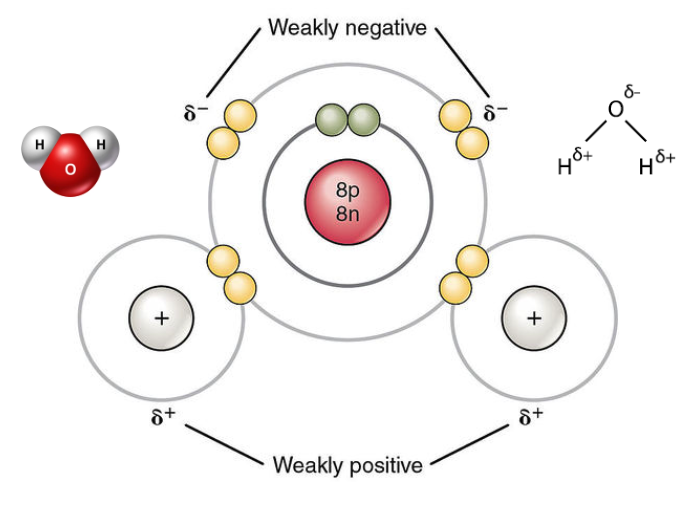
structure of a water molecule
dissolving
when a molecule is separated by another because it is attracted to something
water molecules are polar — where are the electrons polled towards?
the oxygen atom
why do hydrogen bonds form between water molecules?
because the 𝛿+ hydrogen atoms of one water molecule are attracted to the 𝛿− oxygen atom of another water molecule.
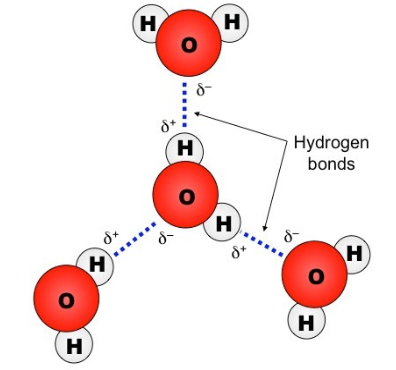
what is the maximum number of H-bonds a water molecule can form with other water molecules?
four
cohesion
the ability of water molecules to form hydrogen bonds with each other, causing them to stick together.
cohesion-tension model
*most widely accepted model for movement of water in vascular plants
transpiration (evaporation) occurs because somata are open. as transpiration occurs, it creates negative pressure called tension/suction.
tension created by transpiration ‘pulls’ water in the plant xylem, drawing it upwards.
cohesion pulls up water molecules in a chain as the top-most water is pulled up and out of the somata.
surface tension
a property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of its molecules.
occurs because the outermost layer of molecules are left with fewer molecules to cling to, instead compensating by establishing stronger bonds with neighbours that they do have contact with, creating an inward force.
adhesion
the attraction of water to other polar or charged molecules.
cellulose
a polymer made of glucose units, present in plant cell walls.
*water forms H-bonds with cellulose because of -OH groups in glucose that water sticks too. this allows structures with lots of cellulose to absorb water.
capillary action
the movement of water through a narrow space, often in opposition to external forces (gravity).
helps bring water from the roots
helps soil retain water
capillarity
the rate at which water is pulled upward from the water table into pore spaces (spaces between soil particles) by capillary action.
*the height to which the water rises depends on the type of soil
**different soils have different capillarity rates
solvent
the liquid in which a solute dissolves
solute
the substance that dissolves in a solvent
solution
a mixture of one or more solutes dissolved in a solvent
solvation
the interaction of a solvent with a dissolved solute
*water forms H-bonds with a solute, forming a hydration shell around it.
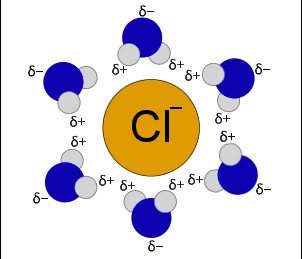
hydrophilic molecules
molecules that attract water (generally soluble)
*polar, with a charge
hydrophobic molecules
molecules that cannot attract water (insoluble)
*non-polar, without a charge
**hydrophobic molecules attract each other, and will clump together when exposed to water
cytosol
the liquid part of the cytoplasm (a structure common to all cells)
catabolic reactions
break down larger molecules into smaller molecules
anabolic reactions
build larger molecules using smaller molecules
why is water necessary for cellular metabolism?
it dissolves reactants and enzymes, so that they can come together for reactions.
how can water be a medium of transport?
dissolved solutes can be transported in solution around the body of an organism.
buoyancy
an upward force applied to an object that is immersed in a fluid
how is buoyancy calculated?
if the buoyant force of the fluid is greater than the objects weight, the object will float.
*buoyancy of a fluid is dependent on its density
density of object > density of fluid = object will float
density of object < density of fluid = object will sink
viscosity
a measure of a fluid’s tendency to flow
what causes / determines viscosity?
the amount of friction the molecules of a liquid experience as they flow over each other.
thermal conductivity
a measure of a material’s ability to move heat across a temperature gradient
what determines thermal conductivity
how easily energy transfers through the material
low conductivity = heat moves slowly through the material (good for insulation)
high conductivity = heat moves rapidly through the material (good for absorbing/transferring heat)
specific heat capacity
the quantity of heat needed to raise the temperature of a chemical, per unit mass.
*water has the highest heat capacity of any liquid.
how has the ringed seal adapted to its environment?
buoyancy in water allows the seal to stay afloat without expending much energy
the seal has adaptations for streamlining as it swims due to the viscosity of water
insulated with blubber to retain body temp due to water having a higher thermal conductivity than air
high specific heat of water provides a stable habitat for the seal to live
scientific name of the ringed seal
pusa hispida
how has the black throated loon adapted to its environment?
buoyancy in water allows the bird to stay afloat without expending much energy
*must expend energy when flying through air though
air is not viscous, which allows the loon to move through it easily when flying
the loon does not lose much body heat when in the air, due to the air having a low thermal conductivity
*the low specific heat of air means that its temperature also changes as rapidly though
scientific name for black throated loon
gavia arctica
extraplanetary object
an object from outside earth’s orbit
the most likely source of water on earth
carbonaceous chrondite asteroids
what are the two reasons that earths surface is able to retain water
being in the goldilocks zone means that the surface is neither too hot nor too cold for the existence of liquid water
gravity
goldilocks zone
the orbital distance from a star that will result in liquid water
(ie the surface temperature is neither too hot nor too cold)
what evidence suggests that water on earth originated from asteroids?
the deuterium to hydrogen ratio in carbonaceous chrondite asteroids is similar to that of the water in earth’s oceans, suggesting a common origin
what are the three circumstances that astrobiologists look for when searching for extraterrestrial life?
luck (less important) - all the right chemicals existing in the same place at the same time
time - earth’s complex life took billions of years to evolve
location - celestial bodies in the goldilocks zone
what are the chemicals that astrobiologists look for when searching for extraterrestrial life?
water — the presence of water is considered the precedent to the search for extraterrestrial life
carbon
nitrogen
phosphorus
sulphur