Physical Separation Methods
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
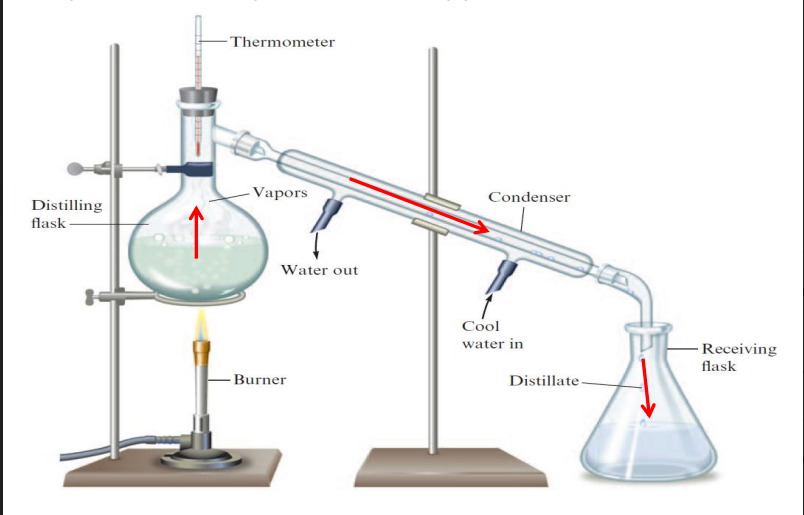
Distillation
Depends on the volatility differences of the components. They vaporize than are condensed back into liquids.
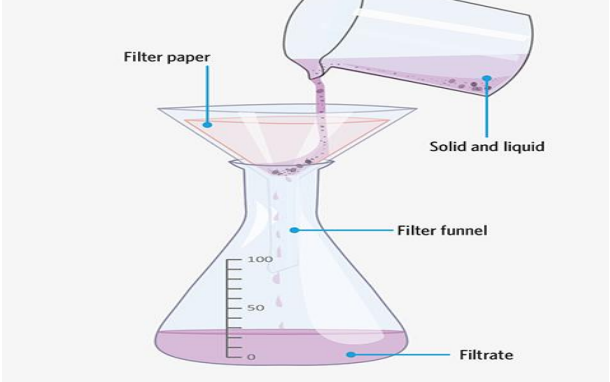
Filtration
Used when a mixture comprises a solid and a liquid where the solid is desired.
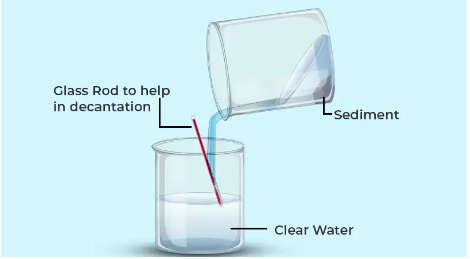
Decantation
Used when a mixture comprises a solid and a liquid where the liquid is desired.
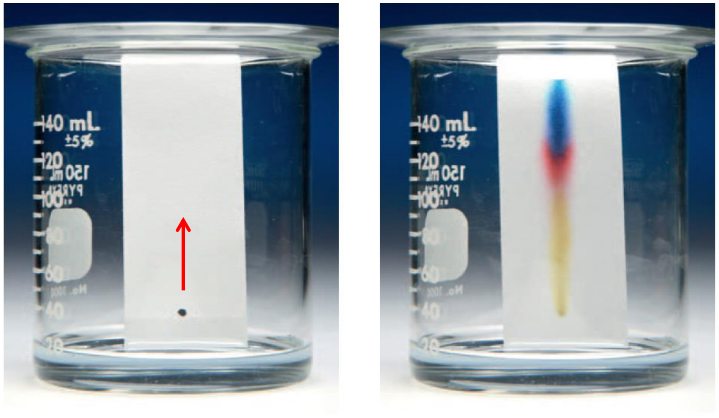
Chromatography
Facilitated by difference in the component’s affinity for the two phases: mobile (liquid or gas) and stationary (solid).
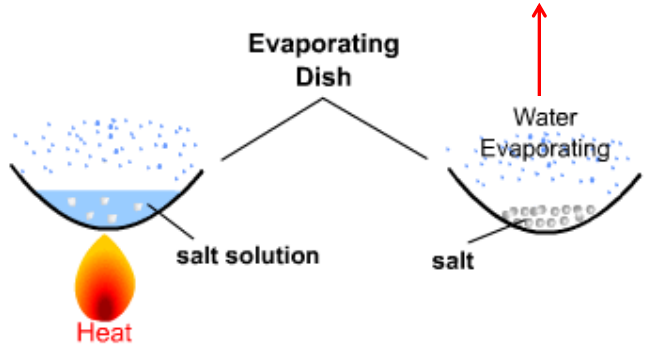
Crystallization
Used to separate dissolved solids (solute) from a solution by evaporating it.
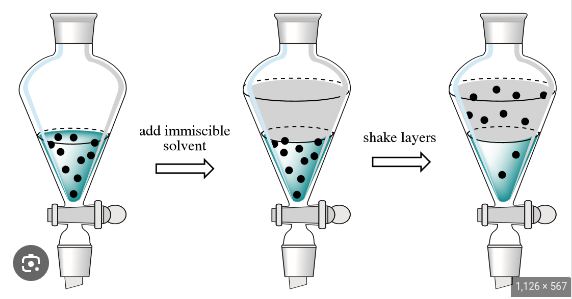
Extraction
A mixture of solids is put into water so that one dissolves and the other doesn’t, thus they are separated.
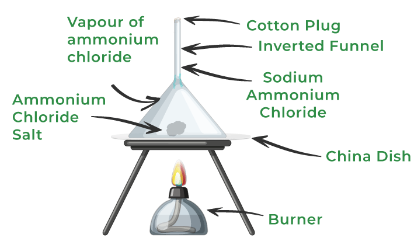
Sublimation
One substance has the ability to go from a solid to a gas without the liquid phase.