Biology - Chapter 1: biological molecules
1/54
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
Monomer
A smaller unit that can create large molecules
Polymer
A molecule made form a large number of monomers joined together
Examples of monomers
Monosaccharides
Amino acid
Nucleotide
Examples of polymers
starch
Glycogen
Cellulose
Protein
DNA
RNA
Examples of monosaccharides
Glucose
Fructose
Galactose
Examples of Disaccharides
sucrose
Maltose
Lactose
Examples of polysaccharides
starch
Glycogen
Cellulose
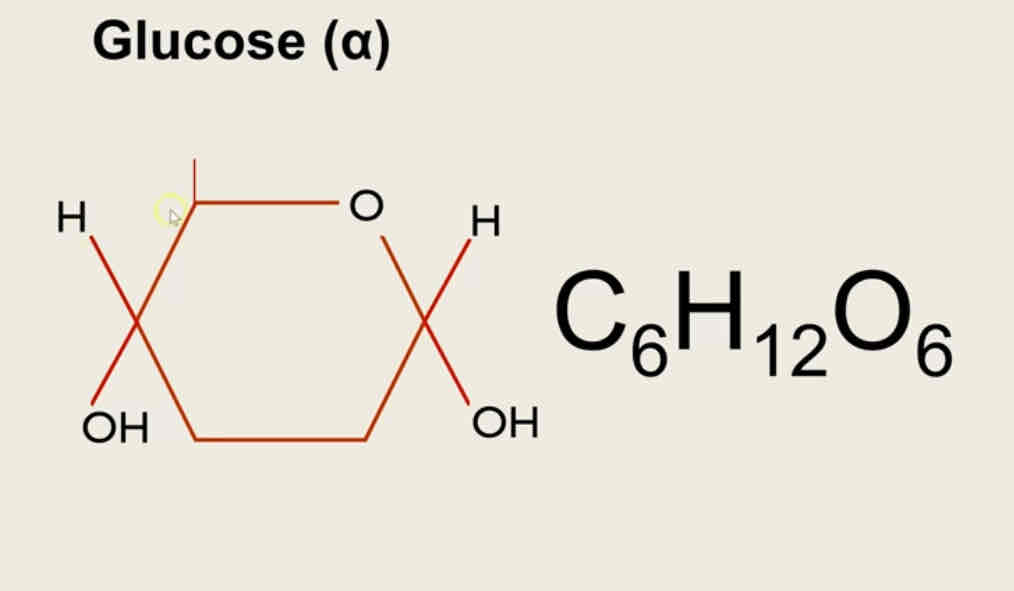
Definition of isomer
Same molecular formula different molecular structure
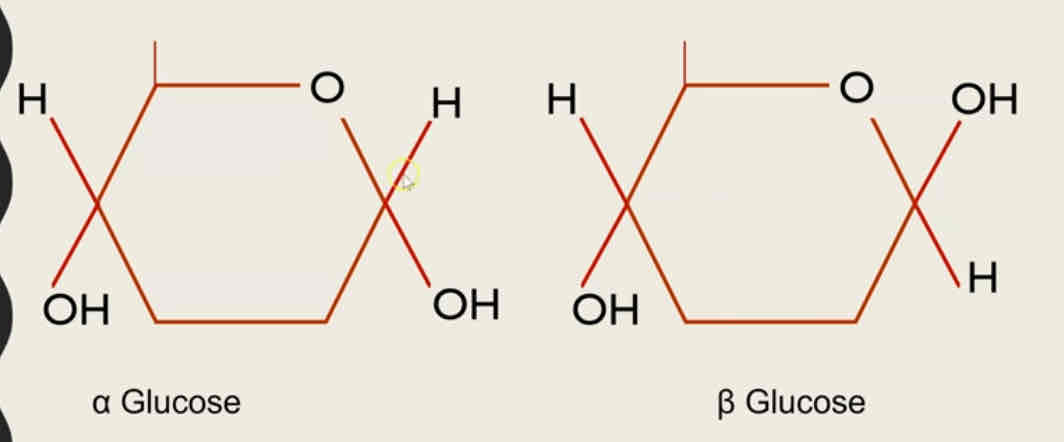
The structure of alpha glucose and beta glucose
The molecular formula is C6H12O6
Definition of disaccharides
Made of two monosaccharides joined together by a glycosidic bond formed via a condensation reaction
disaccharides reaction
Glucose + glucose —> maltose + water
Glucose + fructose —> sucrose + water
Glucose + galactose —> lactose + water
Definition of condensation reaction
Bonding of two molecules fining a new chemical whilst removing water as a product
definition of hydrolysis reaction
Breaking of a chemical bond between two molecules using a water molecule
Definition of polysaccharides
Created by condensation reactions between many glucose monomers
Types of polysaccharides
Starch - in plant - store of glucose
Cellulose - in plant - structural strength in the cell wall
Glycogen - in animals - stores of glucose
Starch
Monomer - alpha glucose
Bonds between the monomers - 1,4 glycosidic bonds in amylose, 1,4 and 1,6 in amylopectin
Function - stores glucose
Location - plant cells (chloroplast)
Structure - made of 2 polymers: amylose - unbranched helix and amylopectin - a branched molecule
How the structure leads to the function?
Helix can be compact to fit a lot of glucose in small spaces
Branched structure increases surface area for rapid hydrolysis back to glucose
Insoluble - won’t be affect water potential
Cellulose
Monomer - beta glucose
Bonds between the monomers - 1,4 glycosidic bonds
Function - structural strength for cell wall
Location - plant cells (cell wall)
Structure - polymer forms long, straight chains. Chains are held in parallel by many hydrogen bonds to form fibrils
How the structure leads to the function?
Many hydrogen bonds provide collective strength
Insoluble - won’t affect water potential
Glycogen
Monomer - alpha glucose
Bonds between the monomers - 1,4 1,6 glycosidic bonds
Function - stores glucose
Location - animal cell (muscle and liver cell)
Structure - a highly branched molecule
How the structure leads to the function?
Branched structure increases area for rapid hydrolysis back to the glucose
Insoluble - won’t affect water potential
triglycerides
formed via the condensation reaction between the one molecule of glycerol and three molecules of fatty acid, producing 3 water molecules and forming an ester bond
r group
it is a fatty acid which can be saturated or unsaturated and no of carbon and hydrogen can vary
saturated fatty acids
the hydrocarbons chain has only single bonds between carbons
unsaturated fatty acids
the hydrocarbons chain consists of at least one double bond between carbons
properties of triglycerides
energy storage. due to the large ratio of energy storing carbon hydrogen bonds compared to the number of carbon atoms, a lot of energy is stored in the molecule
due to the high ratio of hydrogen to oxygen atoms they act as a metabolic water source. triglycerides can release water if they are oxidised. this is essential of animals in the desert, such as camels.
triglycerides do not affect water potential and osmosis. this is because they are large and hydrophobic, making them insoluble in water
lipids have a relatively low mass. therefore a lot can be stored without increasing the mass and preventing movement
emulsion test
add ethanol to sample then distilled water then shake if the a white emulsion appears then lipids are present
phospholipids
made of glycerol molecule and two fatty acid chains and a phosphate group (attached to the glycerol)
the two fatty acids also bond to the glycerol via two condensation reactions resulting in two ester bonds
properties of phospholipids
hydrophilic ‘head’ of a phospholipid can attract with water as it is charged.
due to the phosphate being charged, it repels other fats
the fatty acid chain is not charged. it is known as the hydrophobic ‘tail’ and it repels water, but will mix with fats
have two charged regions, so they are polar.
In water they are positioned so that the heads are exposed to water and the tails are not
this forms a phospholipids bilayer membrane structure which makes up the plasma membrane around cells
what are proteins made up of
amino acids
central carbon with amine group on the left and a carboxyl group on the right and the R group on the top and Hydrogen on the bottom
What are the four structures in a protein
Primary structure
Secondary structure
Tertiary structure
Quaternary structure
Primary structure
The order of the amino acids in the polypeptide chains - this is a polymer
Secondary structure
the sequence of amino acids causes parts of a protein molecule to bend into alpha helix shapes or fold into beta pleated sheets.
hydrogen bonds hold the secondary structure
tertiary structure
the further folding of the secondary structure. to form a uniqure 3D shape. held in place by ionic, hydrogen and disulphide bonds
quaternary structure
a protein made up of more than one polypeptide chainF
enzymes
tertiary structure protein which lowers activation energy of the reactions they catalyse. active site specific and unique in shape due to the specific folded and bonding in the tertiary structure of the protein; can only attach to substrates that are complementary in shape.
models of enzyme action
induced fit, when the enzyme active site is induced or slightly changes shape, to mould around the substrate.
E-S complex occurs, due to the enzyme moulding around the substrate it puts strain on the bonds and lowers the activation energy
factors affecting enzymes:
temperature
pH
substrate concentration
enzyme concentration
inhibitor
temperature affecting enzymes:
temperature too low = not enough kinetic energy for successful collisions between the enzyme and substrate
temperature too high = enzymes denature, the active site changes shape and enzyme - substrate complexes cannot form
pH affecting enzymes:
too high/low = interfere with the charges in the amino acids in the active site. can break the bonds holding the tertiary structure in place and therefore the active site changes shape
therefore the enzyme denatures and fewer enzyme substrate complexes form
substrate and enzyme concentration affecting enzyme:
insufficient substrate = reaction will be slower as there will be fewer collisions between the enzyme and substrate.
insufficient enzymes = active site will become saturated with substrate and unable to work any faster
competition inhibitor
same shape as the substrate
bind to the active site
prevents enzyme - substrate complexes
add more substrate it will flood/out - compete the inhibitior, knocking them out of the active site
non-competitive inhibitors
bind to the allosteric site
causes the active site to change shape
no enzyme-substrate complexes
the substrate can no longer bind regardless of how much substrate is added.
test for starch
add iodine
a positive test observation = solutions turns from orange to dark blue/black
test for reducing sugars
add benedict’s reagent and heat
a positive test observation = solution turns from blue to green, yellow , orange or brick red (the more red the higher the concentration of reducing sugar)
test for non-reducing sugars
following a negative benedict’s test, where the reagent remains blue
add acid and boil - (this is acid hydrolysis)
cool the solution then add an alkali to neutralise
add benedict’s reagent and heat
a positive test observation = solutions turns from blue to orange or brick red (the more red the higher the concentration or reducing sugar)
test for proteins
add biuret
a positive test observation = solution turns from blue to purple
test for lipids
dissolve the sample in sample
then, add distilled water
a positive test observation = a white emulsion forms
DNA
codes for the sequence of amino acids in the primary structure of a protein, which in turn determines the final 3D structure and function of a protein
therefore that cells contain a copy of this genetic code and that it can be passed on to new cells without being damaged
DNA polymer forms a double helix
DNA nucleotide
monomer that makes up DNA = nucleotide.
deoxyribose ( a pentose sugar), a nitrogenous base and one phosphate group
guanine, cytosine, adenine and thymine
polynucleotides
polymer of nucleotides = polynucleotides
via condensation reactions between the deoxyribose sugar and the phosphate group, creating a phosphodiester bond
DNA polymer occurs in pairs joined by hydrogen bonds between bases - creates the double helix
hydrogen bonds can only form between complementary base pairs:
cytosine and guanine
adenine and thymine
RNA
polymer of a nucleotide formed a ribose, a nitrogenous base and a phosphate group
bases: adenine, guanine, cytosine and uracil
relatively short polynucleotide chain and it is single stranded
function: RNA is transfer the genetic code from DNA in the nucleus to the ribosomes. some RNA (rRNA) is also combined with proteins to create ribosomes
DNA replication
before cells divide all DNA must replicate to provide a copy for the new cell
DNA replication = semi - conservative replication
semi - conservation replication
step1:
DNA helicase breaks the hydrogen bonds between the complementary base pairs between the two strands within a double helix. this causes the DNA double helix to unwind
step2:
each of the separated parental DNA strands act as a template. free floating DNA nucleotides within the nucleus are attached to their complementary base pairs on the template strands of the parental DNA
step3:
the adjacent nucleotides are joined together (to form the phosphodiester bond) by a condensation reaction. DNA polymerase catalyses the joining together of adjacent nucleotides
step 4:
the two sets of daughter DNA contains one strand of the prenatal DNA and one newly synthesised strand
evidence for semi-conservation replication
watson and crick discovered the structure of DNA in 1953 helped by Rosalind Franklin’s research on x-ray diffraction
meselson and stahl conducted an experiment which proves DNA replication must be semi - conservation
ATP
a nucleotide derivative, it is a immediate source of energy for biological processes. metabolic reactions in cells must have a constant, steady supply of ATP
ATP made during respiration
ATP can be hydrolysed using ATP hydrolase. releases a small amount of energy
can transfer energy to other compounds. inorganic phosphate released can be bonded onto different compounds to make them more reactive. known as phosphorylation.
five key properties of water
it is a metabolite
an important solvent in reactions
has a high heat capacity, it buffers temperature
high latent heat of vapourisation - providing a cooling effect with loss of water through evaporation
strong cohesion between water molecules; supports water columns and provides surface tension
inorganic ions
occur in solution in the cytoplasm and bodily fluids of organisms
hydrogen ions ——> lower the pH of solutions and impact enzyme function and haemoglobin function. role in chemiosmosis
iron ions ——> a component of haemoglobin in the transport of oxygen
sodium ions ——> involved in the co transport of glucose and amino acids in absorption. in role in generating action potentials
phosphate ions ——> a component of DNA and ATP