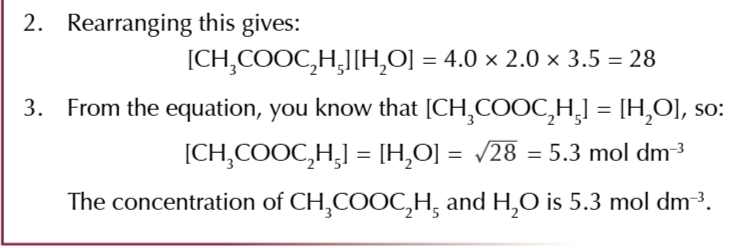1. EQUILIBRIA AND REDOX REACTIONS PART 1
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
as the reactants get used up
the forwards reaction rate slows down
as more product is formed
the reverse reaction rate speeds up
after a while the the forwards and backwards reactions will go at the exact same rate
this means their concentration (though not equal) will not change
there are two types of equilibrium
dynamic
static
the difference between dynamic and static equilibrium
dynamic equilibrium doesn’t need external forces for the backwards reaction to occur
static equilibrium requires external forces like heat for backwards reactions to occur
dynamic equilibrium is reached when
the rate of the forwards reaction is equal to the rate of the backwards reaction
conc of products / conc of reactants remains constant
(whilst in a closed system)
factors that affect the position of equilibrium
concentration
pressure
temperature
Le Chatelier’s principle
if a reaction at equilibrium is subjected to a change in concentration, pressure or temperature, the position of equilibrium will move to counteract the change
catalysts have no effect on the position of equilibrium
they only allow the equilibrium to be reached fast and cannot increase the yield
increasing the concentration of reactant
equilibrium shifts to the right to oppose the increase in reactants, thus increasing the product yield
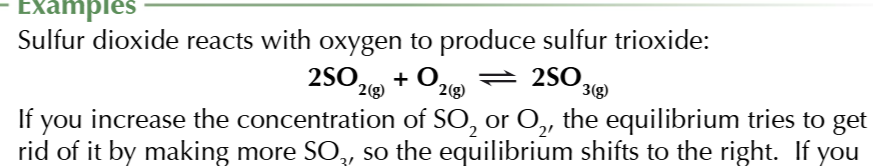
increasing the concentration of product
equilibrium shifts to the left to oppose the increase in products, thus decreasing the yield
increasing the pressure of a reaction
shifts the equilibrium to the side with fewer GAS molecules to reduce the pressure
increasing the pressure of the reaction: 2SO2(g) + O2(g) ⇄2SO3(g)
there are 3 moles of the left and 2 moles on the right THEREFORE the equilibrium will shift to the right to oppose the increase in pressure thus increasing the product yield
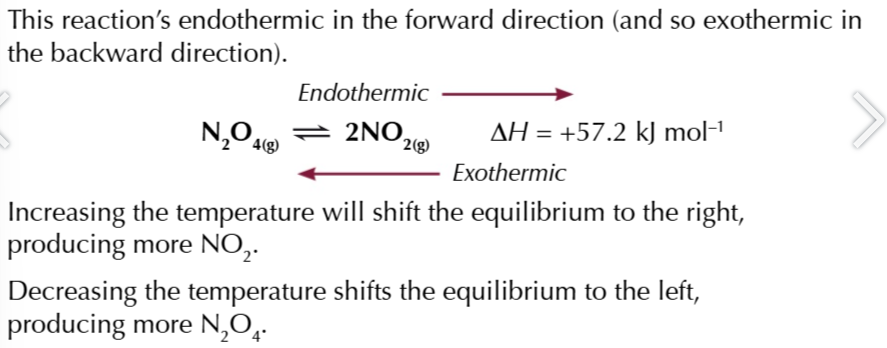
increasing the temperature of a reaction means adding more heat
the equilibrium will shift in the endothermic(+ΔH) direction to absorbed heat
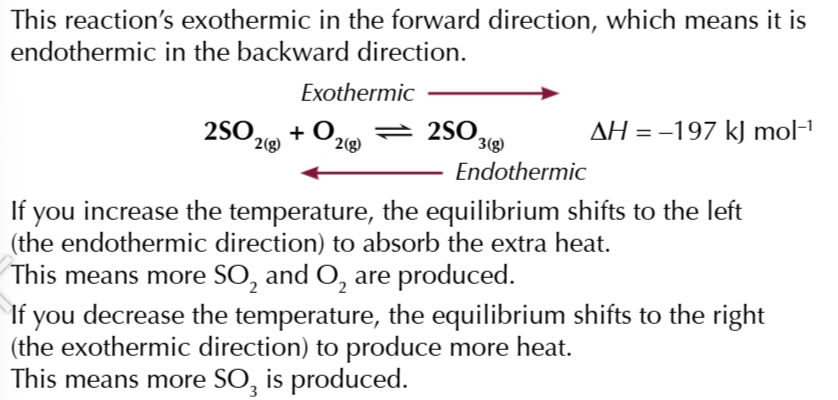
decreasing the temperature of a reaction means removing heat
the equilibrium will shift in the exothermic (-ΔH) direction to release heat
some reversible reactions have distinct colour changes that allow you to follow equilibrium reactions
[Cu(H2O)6]2+ + HCl ⇄ [CuCl]2-
![<p>for the reaction<span style="color: #007bff"> [Cu(H2O)<sub>6</sub>]<sup>2+</sup></span> + HCl ⇄ <span style="color: #689b00">{CuCl}<sup>2-</sup></span> if the solution is <span style="color: #00b2ff">blue</span></p>](https://knowt-user-attachments.s3.amazonaws.com/3b7ae915-49bd-4bbe-b2ef-120c12c9aabd.png)
for the reaction [Cu(H2O)6]2+ + HCl ⇄ {CuCl}2- if the solution is blue
the position of the equilibrium must lie on the left so there will be more reactants than products
![<p>for the reaction <span style="color: #00d4ff">[Cu(H2O)<sub>6</sub>]<sup>2+</sup></span> + HCl ⇄ <span style="color: #79c500">{CuCl}<sup>2-</sup> </span>if the solution is <span style="color: #7bb900">greeny-yellow</span></p>](https://knowt-user-attachments.s3.amazonaws.com/2f920515-4f32-4147-8c69-e20de2249b2e.png)
for the reaction [Cu(H2O)6]2+ + HCl ⇄ {CuCl}2- if the solution is greeny-yellow
the position of the equilibrium must lie of the right so there will be more products than reactants
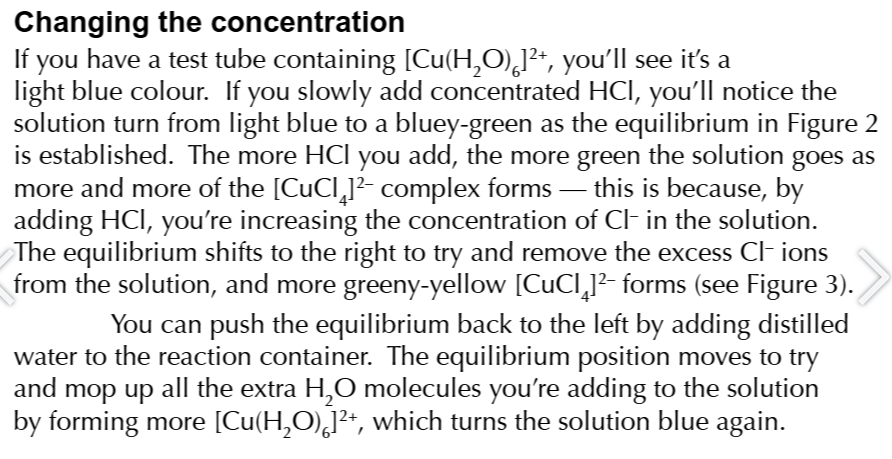
changing the concentration
changing the temperature
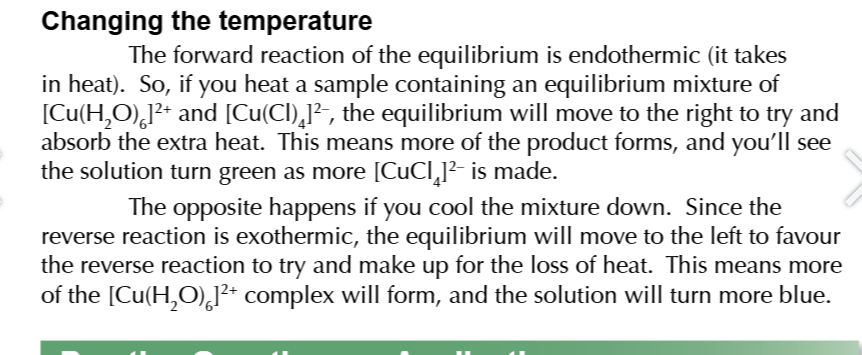
compromise conditions in industry
companies have to think about the
cost to run a reaction,
how much money they can make from it,
the time taken for a suitable yield to be produced etc
C2H4(g) + H2O ⇄ C2H5OH(g), ΔH=-46kJ mol-1 industrial conditions to make alcohol from alkene:
pressure: 60-70 atm
temperature: 300℃
phosphoric acid catalyst
300℃
forwards reaction is exothermic so a low temperature is will give a better yield HOWEVER it will also decrease the rate of reaction, 300℃ is a compromise between maximum yield and a faster reaction
60-70 atm
higher pressures favour the forwards reaction since the equilibrium will shift to the side with less moles HOWEVER an extremely high pressure is expensive to produce as stronger pipes and containers to withstand high pressure will be required so 60-70 atm is a compromise between maximum yield and minimum expense
because only a small proportion of ethene reacts each time the passes pass through the reactor
to save money and raw materials the unreacted ethene is separated from the ethanol and recycled back into the reactor so 95% of ethene is eventually converted to ethanol


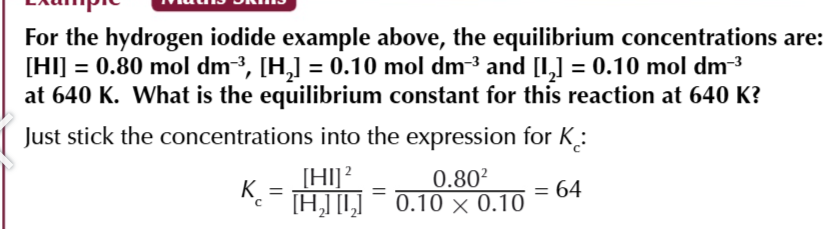
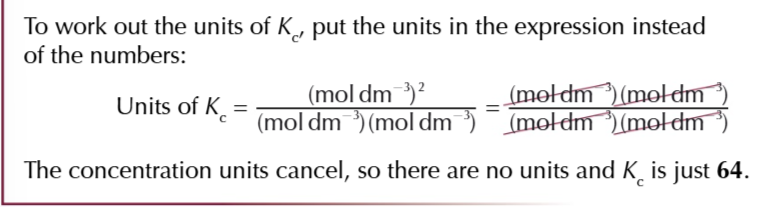
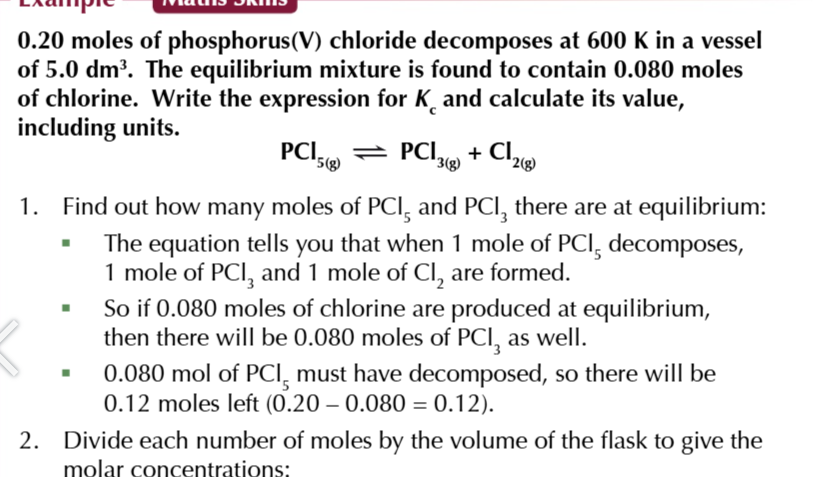
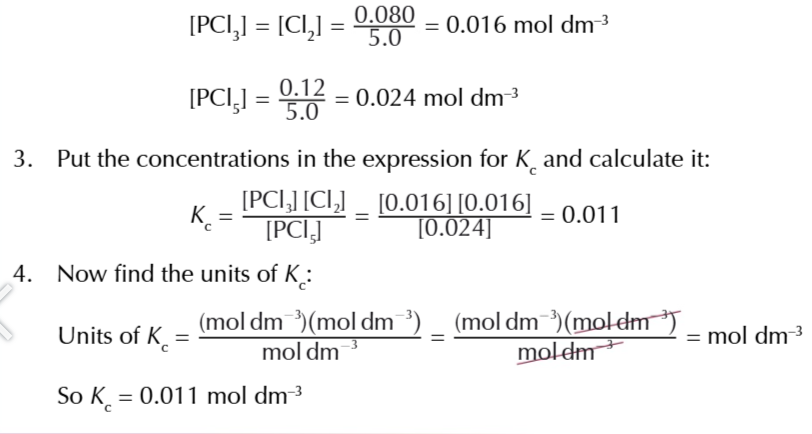
equilibrium constant
the ratio worked out from the concentrations of the products and reactants after equilibrium is reached
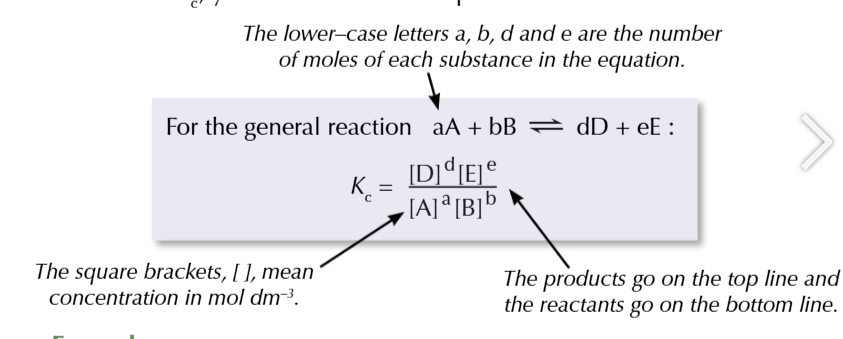
the value of Kc
will only be true for that particular temperature, no other factors affect Kc