Suspensions and Emulsions
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
41 Terms
What is a molecular dispersion?
True Solution (generally small mols)
What is a colloidal dispersion?
1 nm – 0.5 mm (one or two phases)
A mixture containing small particles that can maintain a uniform dispersion (remain suspended) without a suspending agent.
NO SHAKING REQ.
What is a coarse dispersion?
>0.5 mm (Two phases)
A mixture containing particles that maintain a uniform dispersion with the addition of a suspending agent.
SHAKE WELL
What are lyophilic colloids?
SOLVENT LOVING
– Have a strong affinity (adhesive interactions) for the solvent leading to spontaneous formation of collodial dispersions
– Relatively easy to prepare – solvation or hydration
– High concentrations are generally more viscous and can form gel
What are lyophobic colloids?
SOLVENT HATING
– Little to no affinity (adhesive interactions) for the solvent
– Requires special means of formulation to form dispersions that are relatively stable (sonication, homogenization,)
– Generally involve dispersing phases which are incompatible.
Brownian Movement vs. diffusion
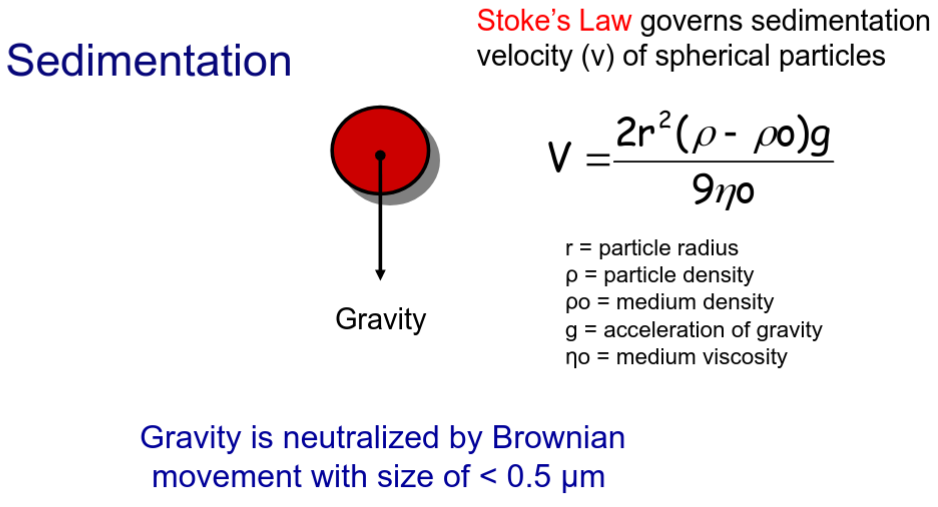
Gravity is neutralized by Brownian movement with size of < 0.5 μm
What are the 3 properties of dispersions?
Kinetic (how it moves)
Viscosity (What it moves through)
Sedimentation (how fast it falls)
Why do we need to think about viscosity for disperisons?
Resistance to flow:
Consider the colloid concentration, shape of colloid, and adhesive interactions with the solvent and cohesive interactions with other like molecule
How does stokes law play a part in dispersions?
SEDIMENTATION VELOCITY! of spherical particles
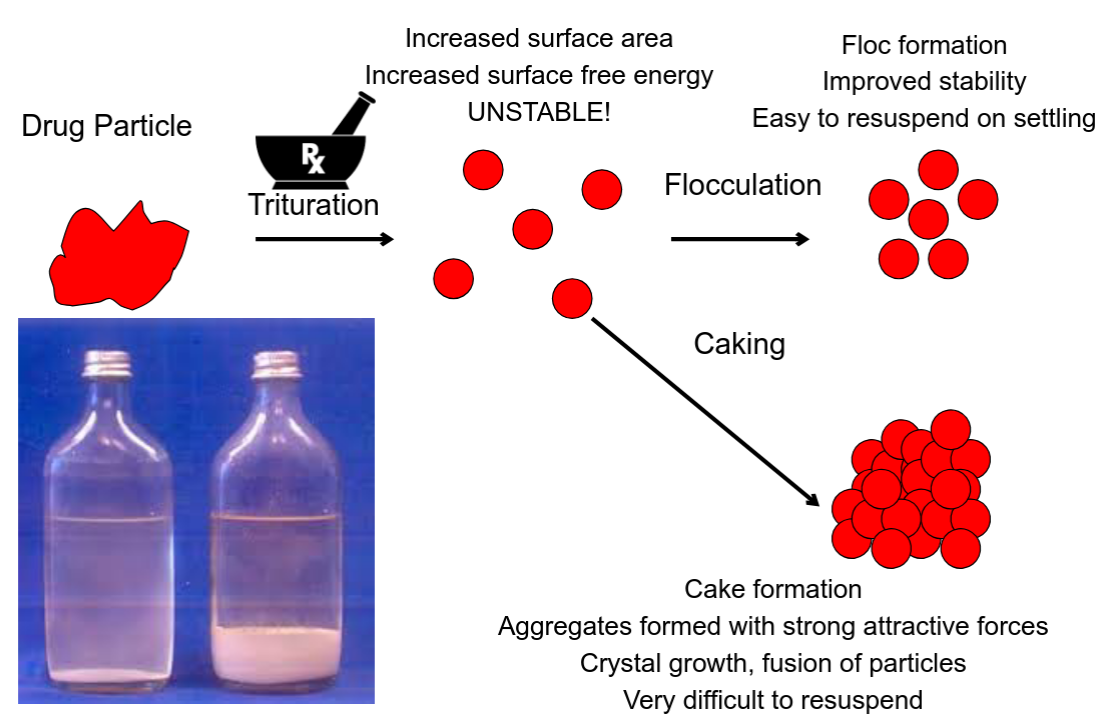
What is aggregation?
Collection of particles into groups held together by
strong interactions
What is coagulation?
Closely aggregated particles that are permanently
bound to each other
What is flocculation?
Aggregates held together by weak interactions
that are readily broken.
What determines a system’s tendency to either aggregate, coagulate, or flocculate?
It depends on the forces of interaction between the particle
Three groups of forces:
Electrical forces of Attraction
Electrical forces of Repulsion
Forces of Solvation
What stabilizes lyophobic dispersions?
– Brownian motion and/or sedimentation results in interactions between particles
– Stabilized by electric double layer forces only!
– Very sensitive to electrolytes, alters electrical double layer forces. (Zeta potential)
What stabilizes lyophilic dispersions?
– Stabilized by the electric double layer and forces involved in solvation
– Generally stable to electrolytes. May be “salted-out” with high concentrations. Due to electric layer alterations and Desolvation.
– Addition of less polar solvents, such as alcohols, can also lead to desolvation. Eg: Precipitation of DNA using 70% Ethanol
– Mixing oppositely charged colloids, altering pH or adding complexing ions can lead to Coacervation. Used in Film coating and microencapsulation!!
What is Coacervation?
the separation of a colloid-rich layer from a lyophilic sol on addition of another substance
What is a suspension?
• A Two phase, hetergenously dispersed system composed of finely divided, undissolved solute/drug dispersed into a liquid continuous phase
• Classified as coarse and lyophobic dispersions
– Due to size of the particles and interaction with solvent
Why and how do we use suspensions?
Used for Oral administration, External application as lotions or for otic/opthalmic/nasal instillation, and Injectables
• poorly soluble
• more stable in suspension
• control the rate of drug availability. Dissolution rate controlled
• taste
• dosing flexibility and easy administration
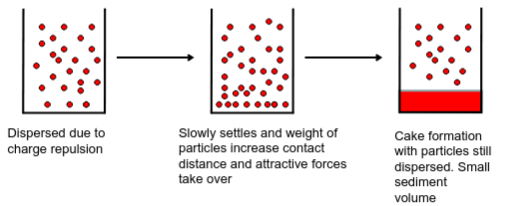
Cake vs. Floc
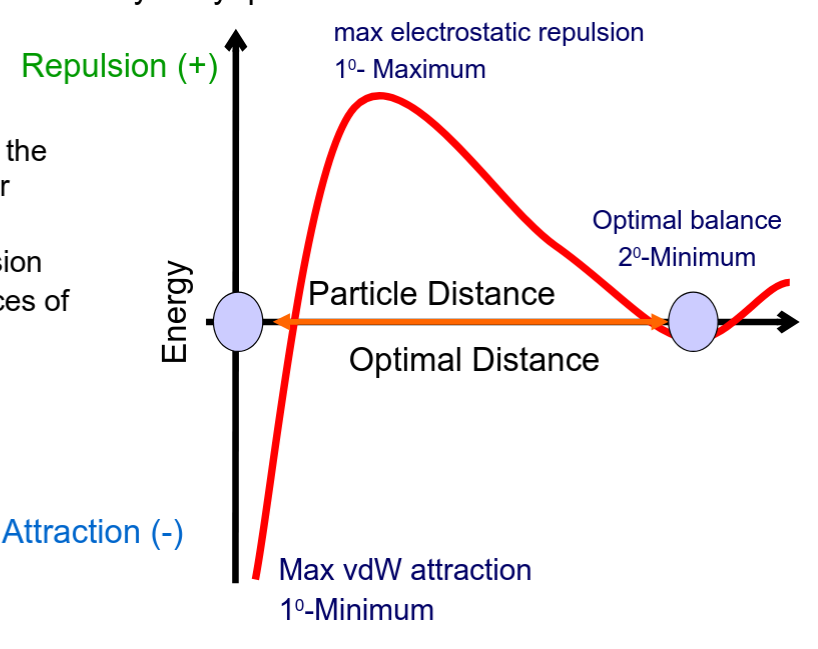
What is the DLVO theory?
A theory for the stability of Lyophobic sols.
It considers the forces of interactions for Lyophobic systems as:
• Electrostatic repulsion
• van der Waal’s forces of attraction (IMF’s)
How are electric fields formed?
Since counter ions can move around it can go around and “neutralize“ the dominant charge
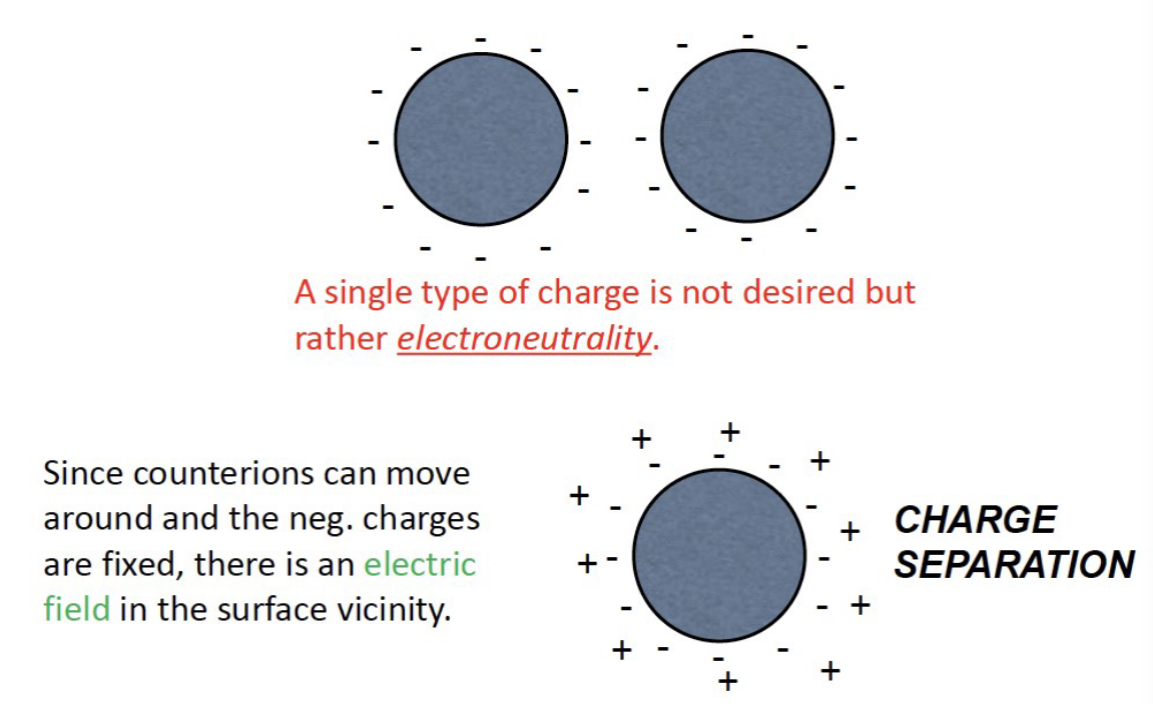
What can we take away from the DLVO theory?
High electrostatic repulsion results in deflocculated particles. RESULTS IN CAKE!
Optimal balance in the forces of attraction and repulsion leads to loosely aggregated particles called Flocs
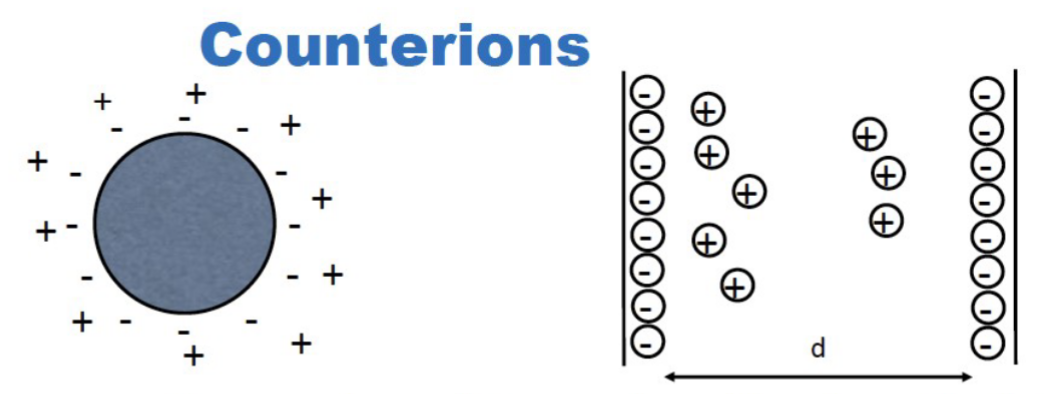
What do counter ions do?
They act as screening electrolytes, they screen the repulsive effects of the fixed charges on the surface.
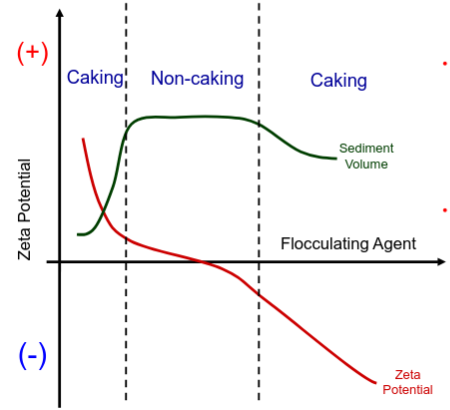
How do we ensure flocculation?
• FLOCCULATING AGENTS changes zeta-potential of the particles (it can be electrolyte, charged surfactant or charged polymer adsorbing on a surface).
• If the absolute value of the zeta-potential is too high the system deflocculates because of increased repulsion and the dispersion cakes
Conditions are altered so that drug particles occupy the region of 2’ on the DLVO diagram. Altering the charge of the drug particle by use of a flocculating agent to adsorb to the drug surface.
Can also alter pH of the media to change the degree of charge.
Another approach is “bridging”, uses a high molecular weight polymer that prevents caking by bridging between particles. Particles remain flocculated because they are trapped in a complex polymer network.
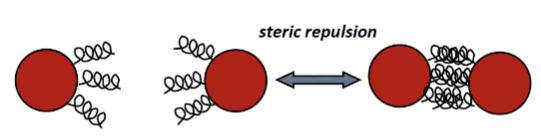
What is a steric repulsive barrier?
A repulsive barrier by adsorbing a long chain polymer or other large macromolecules onto the surface.
e.g. polymer chains
nonionic polymers like PEG work well:
not sensitive to surface chare and salt conc
works well in non aqueous media
works in concentrated dispersions
How do we control settling?
Reducing particle size and altering medium viscosity by dissolving lyophilic colloids, called “suspending agents or thickening agents” will reduce sedimentation velocity
Suspending agents prevent particle
contact, to reduce caking
What properties do thixotropic agents have?
Thixotropic suspending agents have a high viscosity under low shear and a lower viscosity under high shear.
Suspending agents should have thixotropic properties!
KETCHUPPPPPP
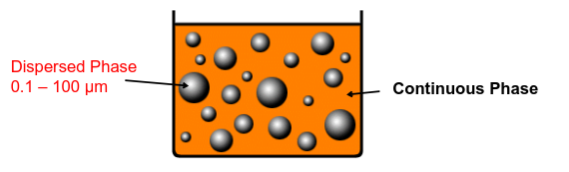
What are emulsions?
A Two phase system, consisting of at least two immiscible liquids with one liquid hetergenously dispersed as fine globules/droplets in the other liquid phase, with the aid of an emulsifying agent
Classified as coarse and lyophobic dispersions
– Due to size of the particles and interaction with solvent
What are the different phases in emulsions?
• Phase that is presented as a fine droplet is called the dispersed phase or internal phase.
• The phase in which the dispersed phase is suspended in is called the continuous phase or external phase
Examples of different emulsion combinations
Oil in water
Water in oil
Oil in water in oil
Water in oil in water
Why and how do we use emulsions?
Used for Oral administration, opthalmic, External application as lotion, cream, or ointment base or in Injectables (IV)
Administration of oil liquids
mask Nasty Taste
hydrophobic drugs
Oral absorption via lymphatic system
How do we keep an emulsion stable?
We want it to t retain a uniformly distributed dispersed phase throughout the continuous phase
They require an Emulsifying agent, to reduce interfacial tension between the two phases, impart charge, and act as a steric barrier
Emulsions are thermodynamically un-stable and are formulated to maintain Kinetic stability
What do emulsifying agents do?
• Adsorb at the Liq – Liq interface between the two phases and stabilize emulsions by either:
1. Decreasing interfacial tension
2. Forming a physical/steric barrier around the dispersed phase
3. Impart electrostatic charge – Electric double layer! (DLVO)
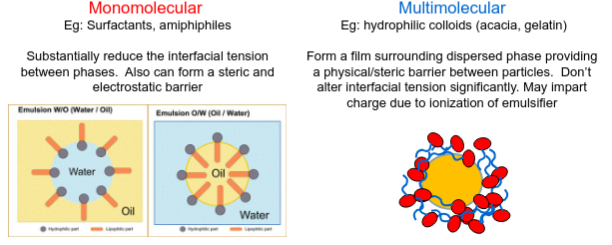
What are the 2 types of emulsifying agents?
Monomolecular (Eg: Surfactants, amiphiphiles)
Substantially reduce the interfacial tension between phases. Also can form a steric and electrostatic barrier
Multimolecular (Eg: hydrophilic colloids (acacia, gelatin))
Form a film surrounding dispersed phase providing a physical/steric barrier between particles. Don’t alter interfacial tension significantly. May impart charge due to ionization of emulsifier
What can the IN-stability of an emulsion can be classified as?
1. Creaming
2. Coalescence
3. Phase inversion
4. Misc. physical/chemical changes (Bacteria, degradatio
What is cracking/breaking?
Any change in the interfacial tension or film between the two phases can lead to the separation of an emulsion into it’s constituent part
What is creaming?
Many emulsions “cream” upon standing. Due to the density differences between the phases.
– Oil in water cream UP
– Water in oil cream Down
Creaming is not generally a sign of instability, since
globules can be re-dispersed However, creaming can lead to coalescence!
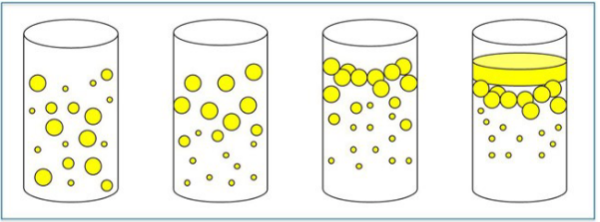
How do we control creaming?
The rate of creaming can be decreased by:
– Reducing globule size
– Decrease the density difference between the phases
– Increase the viscosity of the continuous phase
• Suspending agents!!
Think of Homogenized Milk
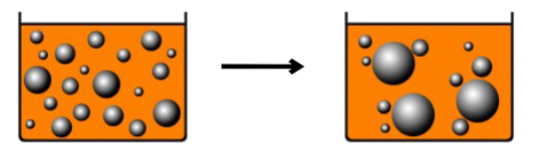
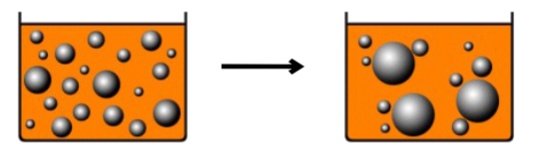
What is Coalescence?
An irreversible process where dispersed globules that have flocculated and/or creamed can fuse together, resulting in larger globules.
How can Coalescence happpen?
Due to a loss of the stabilizing forces surrounding the dispersed phase. i.e. loss of DLVO stabilizing forces or physical removal of emulsifiers
– Temperature
– Bacteria
– Incompatible Chemicals: preservatives, drugs, ions, etc.
What is Phase Inversion?
Process of inter-conversion between O/W to W/O emulsion, or vice versa. (Dry gum method/wet gum (4:2:1 or continental))
Phase volume ratio or amount of dispersed phase can constitute >50% of the total volume.
– Alterations in the phase volumes can lead to phase inversion
• Also can occur due to alterations in the emulsifier HLB value. Eg: added ions