Patho: Exam 2- Fluids and Electrolytes Acids and Bases
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
136 Terms
Intracellular Fluid (ICF)
fluid in the cells, females have less because of the tissue makeup (more subcutaneous and fatty tissue)
Females (more subcutaneous and fatty tissue)
Who has less ICF? Females or Males?
2/3 of total body water
What percentage of total body fluid does intracellular fluid make up?
Extracellular fluid (ECF)
fluid outside the cells; includes intravascular and interstitial fluids
1/3 of total body water ( intravascular and interstitial fluids)
What percentage of total body fluid does extracellular fluid make up?
Interstitial Fluid
fluid in the spaces between cells; is a clear, watery substance that fills the spaces between cells and tissues thorough the body, fluid in the spaces of the cells
intravascular fluid
fluid within blood vessels (plasma)
Plasma
the yellowish, liquid part of blood that transports blood cells
transcellular fluid
- The fluid that is contained within specialized body compartments such as cerebrospinal, pleural, and synovial cavities
- in the epithelial cavities of the body
CSF, synovial fluid, aqueous humor (in the eye), saliva, GI fluid, Pleural fluid, Peritoneal fluid
What are some examples of where you would find transcellular fluid?
60%
In adults what percentage of fluid makes up body weight?
approx 75-80% (newborns)
approx 65% (infants and younger children)
In pediatrics, what percentage of fluid makes up body weight?
infants and young children
Who is most at risk for significant risk in changes of body fluids, think dehydration?
-they have less subcutaneous tissue
- decrease in muscle mass
-decrease in renal system
-less total body water
-diminished thirst perception
What happens in the aging adult tissue an muscle wise?
1. Osmosis - Water moves from areas with more water (low solutes) to areas with less water (high solutes) to balance things out.
2. Hydrostatic Pressure - The heart pushes blood through blood vessels, forcing water out into the spaces around cells.
3. Oncotic (Osmotic) Pressure - Proteins in the blood pull water back into the bloodstream to keep fluid levels balanced.
4. Filtration at the Arterial End: Blood pressure (hydrostatic pressure) forces fluid and small solutes out of the capillaries into the interstitial space.
5. Reabsorption at the Venous End: As blood moves through the capillary, hydrostatic pressure drops, but oncotic pressure remains relatively constant. This difference pulls water back into the capillaries.
Describe the movement of water between the fluid compartments.
Hydrostatic pressure
the pressure within a blood vessel that tends to push water out of the vessel; a type of filtration that happens in the body where water is pushed out of our capillary system
osmotic/oncotic pressure
where pressure is pulling water into the capillaries; the movement of fluid into the capillary from the interstitial space
water, sodium, glucose
What are some examples of things that move freely across different capillary membranes?
to help create osmotic pressure
Why does protein NOT cross the capillary membrane easily?
Starling forces
a sum of the forces generated by hydrostatic and osmotic pressures; results in a greater attraction of fluid to one side of a membrane
forces act together and cause net filtration; forces that determine where the net is filtration or reabsorption
Capillary hydrostatic pressure
outward force, aka blood pressure, facilitates the movement of water form our capillaries into the Interstitial spaces
capillary oncotic pressure
through osmosis helps to attract or pulls water from the capillaries to the interstitial spaces into the capillary system
c. Albumin
Capillary oncotic pressure is primarily determined by which of the following molecules?
A) Glucose
B) Sodium
C) Albumin
D) Water
Albumin
Another word for a protein in the blood that helps maintain fluid balance by pulling water into the bloodstream.
It is the main protein responsible for capillary oncotic pressure and prevents excess fluid from leaking into tissues.
proteins, hormones, vitamins
What does Albumin help transport?
Filtration
The movement of fluid out of the capillaries into the interstitial spaces (the area around cells) due to hydrostatic pressure(the force exerted by blood inside the capillaries). This process helps deliver oxygen and nutrients to tissues.
edema
The accumulation of excess fluid within the interstitial spaces (the area around cells).
blockage in lymph system
increase in capillary permeability
increase in hydrostatic pressure
a decrease in plasma oncotic pressure
what can edema be caused by?
0+ on edema scale
no pitting edema
1+ edema scale
Mild pitting edema. 2mm depression that disappears rapidly
2+ edema scale
moderate pitting edema, 4mm depression that disappears in 10-15 seconds
3+ edema scale
moderately severe pitting edema, 6mm depression that may last more than 1 minute
4+ edema scale
Severe pitting edema. 8mm depression that can last more than 2 minutes.
decreased, accumulation of fluid
a ___________ level of protein can cause what?
pitting edema
when you push in and the skin goes down and an be measure from 0-4+, interstitial fluid exceeds to absorption capacity of our tissues, the fluid stays there
Non-pitting edema
Fluid accumulation that is "harder" and not compressible when pressure is applied;
when the fluids and proteins leak into that interstitial space
Isotonic IV fluid
normal saline (0.9% sodium chloride), helps maintain fluid balance in the body. Another example is Lactated Ringer's solution, which contains electrolytes and buffers like sodium, potassium, calcium, and lactate.
This fluid adds fluid to the body and offers hydration
hypotonic IV fluid
such as 0.45% sodium chloride (half normal saline), is used for rehydration by shifting fluid into the intracellular space, helping to hydrate cells.
to rehydrate cells by shifting fluid from the intravascular space into the intracellular space.
This helps treat cellular dehydration, which can occur in conditions like hypernatremia or diabetic ketoacidosis.
What is the purpose of a hypotonic IV Fluid?
Hypertonic IV Fluid
contains a higher concentration of solutes than the body's cells, causing fluid to move from the intracellular space into the intravascular space.
Examples include 3% sodium chloride and 5% dextrose in normal saline (D5NS), which provide a high concentration of sodium or glucose.
- Severe hyponatremia (low sodium levels) to help restore sodium balance.
- Cerebral edema to reduce brain swelling by drawing fluid out of brain cells.
- Hypovolemic shock to expand intravascular volume when other fluids are insufficient.
- you would want to use this fluid someone with excess fluid,
Who would we want to give a hypertonic IV fluid to?
the concentration of the fluid (osmolality) of the ECF is elevated above normal, cause- increase of extracellular fluid sodium, if there is a deficient of ECF or extra water
Describe the changes in a hypertonic IV solution.
Hypernatremia
is a condition characterized by an elevated sodium level in the blood, typically caused by either an excessive intake of sodium or a loss of water, leading to a higher concentration of sodium in the bloodstream
hyperchloremia
increase of chloride, can be cause by an excesses of sodium or a decrease of bicarbonate
hypotonic alterations
when the osmolality of our ECF is less then normal leading to a shift of water into cells, causing them to swell.
This is typically due to hyponatremia (low sodium levels) or excess free water intake or retention
Hyponatremia
sodium levels are low and this case water to move into the cells and can happen because there is not enough sodium or there is a dilution of the body's sodium levels because of excess fluid or water; we want to think about restricting fluid intake
This imbalance can lead to cellular swelling, particularly in the brain, causing neurological symptoms.
what can hyponatremia lead to and cause?
Hypochloremia
s a condition characterized by low chloride levels in the blood (serum chloride <97 mEq/L).
can be caused by an elevation in bicarbonate levels in the blood, a condition known as metabolic alkalosis
What can hypochloremia be caused by?
Most common electrolyte in cellular fluid
it regulates intracellular fluid osmolality
maintains the resting membrane potential
if needed and necessary for glucose and glucagon to be deposited or stored in the liver and muscle cells, important for the maintenance of our normal cardiac rhythm and the contraction of muscles in general
What is potassium?
Through the kidneys, aldosterone secretion, insulin secretion, general changes in pH
How does the body try to maintain homeostasis of potassium?
Potassium Adaption
refers to the body's ability to gradually adjust to changes in potassium levels, particularly when potassium levels increase or decrease slowly over time. The body can accommodate small changes in potassium more effectively when the change happens gradually, allowing cells and organs (like the kidneys and heart) to adapt.
rapid changes in potassium levels, even small ones, can cause severe effects, especially on the cardiac system
Hypokalemia
deficient potassium in the blood
low potassium, total loss of body [potassium, reduced intake, a shift of potassium in the cells, increase loss
Bananas and potatoes
What are some foods that have potassium?
less than 3.5 mEq/L.
what is the lab value for hypokalemia?
3.5 to 5.0 mEq/L,
what is the normal lab value for potassium?
depend on rate and severity
Generalized skeletal weakness
cardiac dysrhythmias
glucose intolerance
ability to impair the concentration of the urine
What are some signs and symptoms of hypokalemia?
hyperkalemia
excessive potassium in the blood; increase in potassium, when the potassium is greater then 5.5 meq per liter
Excessive potassium intake.
Shift of potassium from the intracellular fluid (ICF) to extracellular fluid (ECF).
Decreased renal excretion of potassium.
Certain medications that decrease potassium excretion.
what are the causes of hyperkalemia?
Increased neuromuscular irritability, restlessness, and diarrhea.
Intestinal changes and cramping.
what are some mild changes that can happen in hyperkalemia?
Excessive potassium consumption (e.g., dietary or supplements).
Medications like MiraLAX or other laxatives.
Conditions like bulimia or eating disorders that may involve laxative abuse.
what are some causes for some of the mild changes that can occur in hyperkalemia?
Calcium
a Metabolic Processes and is found mainly in the bones, 99 percent in the bones, we need it for the metabolic processes in the body, bones, teeth
important in blood clotting, for hormone secretions, helps with nerve transmission, helps w/ muscle contraction
what is calcium important for?
8.5 to 10.5 mg/dL
what is the normal lab value for total calcium?
parathyroid hormone (PTH)
is a hormone produced by the parathyroid glands, which are small glands located behind the thyroid gland in the neck. PTH plays a crucial role in regulating the body's calcium and phosphate levels.
30 to 50 ng/mL
what is a normal lab value for vitamin d3?
Vitamin D3
also known as cholecalciferol) is a fat-soluble vitamin that is essential for maintaining healthy bones and regulating calcium and phosphorus levels in the body
Calcitonin
Vitamin D3
Parathyroid Hormone
What three things work together to help control phosphate absorption and secretion?
Calcitonin
Lowers blood calcium levels
Hypercalcemia
an abnormally high level of calcium in the blood
Excessive vitamin D intake.
Bone metastasis (spread of cancer to bones).
Overactive parathyroid glands (hyperparathyroidism).
Certain cancers or diseases affecting calcium metabolism.
what are some causes of Hypercalcemia?
Fatigue
Weakness
Lethargy
Constipation
Kidney stones (due to high calcium levels leading to calcium deposits in the kidneys)
Impaired renal function (due to kidney damage from excess calcium)
Dysrhythmias (abnormal heart rhythms due to the effect of high calcium on heart function)
Bone pain and osteoporosis (due to increased calcium release from bones, weakening bone structure)
what are some manifestations of hypercalcemia?
less than 8.5 mg/dL.
what is the lab value for hypocalcemia?
Chvostek's sign
Contraction of the ipsilateral facial muscles elicited by tapping the facial nerve just anterior to the ear
Contradiction of the mouth alone occurs in 10-30% of normal individuals

Trousseau's sign
A sign of hypocalcemia . Carpal spasm caused by inflating a blood pressure cuff above the client's systolic pressure and leaving it in place for 3 minutes.
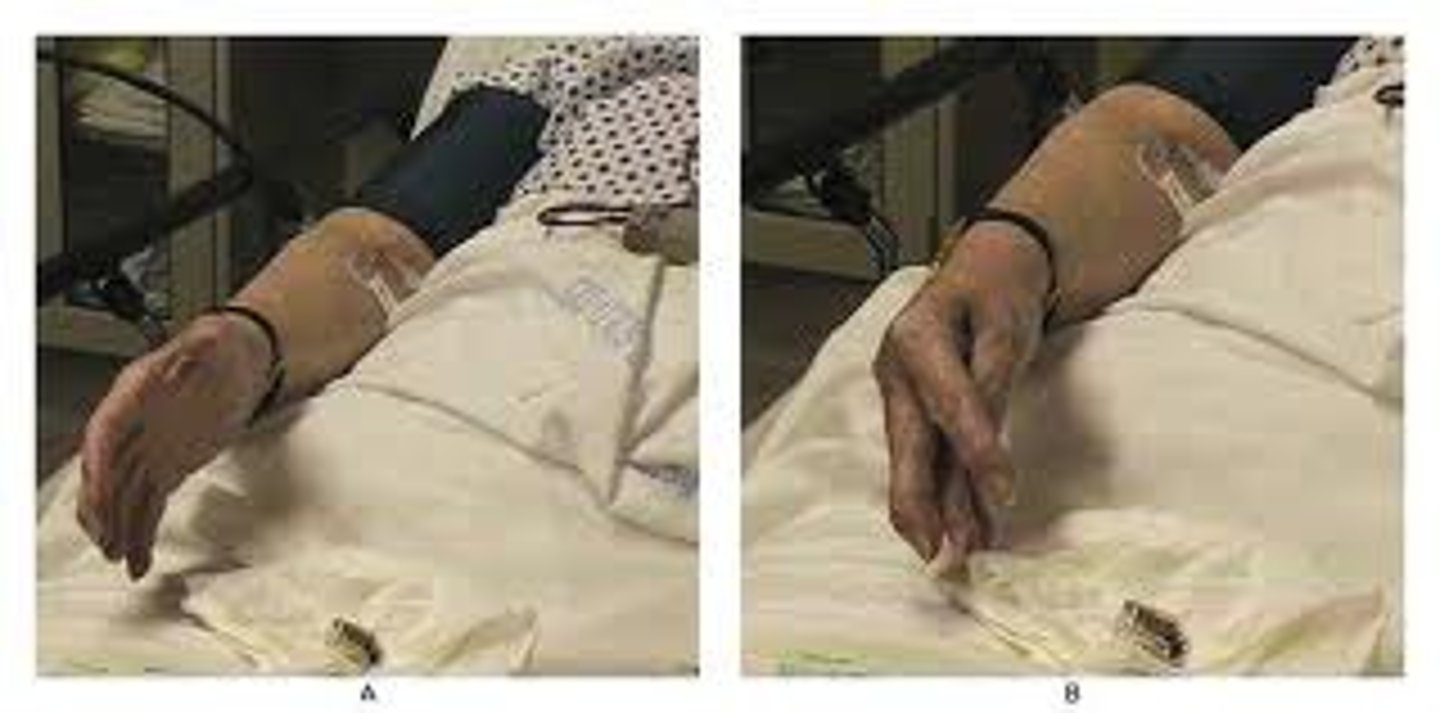
Poor absorption of calcium (e.g., malabsorption disorders).
Calcium deposition in bones or soft tissues, reducing available calcium in the bloodstream.
Decreased parathyroid hormone (PTH) levels.
Insufficient vitamin D levels, which impair calcium absorption.
what are the causes for hypocalcemia?
Increased neuromuscular excitability, leading to symptoms like muscle cramps or spasms.
Seizures or convulsions due to nerve hyperactivity.
Cardiac arrest in severe cases, as calcium plays a critical role in cardiac muscle contraction.
ECG changes: Thin or flattened T waves on the PQRST complex, and a prolonged PR interval due to delayed conduction in the heart.
what are some manifestations for hypocalcemia?
Phosphate
acts as a buffer in acid-base regulation within the body, provides energy for muscle contraction, and provides energy for muscle contraction, most in the bone
2.5 to 4.5 mg/dL.
What is the normal lab value for phosphate?
Hyperphosphatemia
excessive phosphate in the blood
above 4.5 mg/dL
what is the normal lab value for Hyperphosphatemia?
It often occurs in people with acute or chronic renal failure, especially when there is significant loss of glomerular filtration.
What are some manifestations for Hyperphosphatemia?
Hypophosphatemia
Low phosphate levels in the blood.
below 2.5 mg/dL.
what is the lab value for Hypophosphatemia?
intestinal malabsorption and an increase in renal excretion
What are some causes for Hypophosphatemia?
Magnesium
Can act as a cofactor in Intracellular enzymatic reactions , Interacts with calcium on a cellular level, increase neuromuscular activity
1.8-3.0 mEq per liter
what is the normal lab value for magnesium?
Hypermagnesemia
excess of magnesium in the extracellular fluid; typical cause is renal failure
greater then 3.0
what is the lab value for Hypermagnesemia?
can use skeletal smooth muscle contractions, diminished deep tendon reflex, generalized muscle weakness,
we will see nausea and vomiting, might be excess nerves functions, hypotension, bradycardia, and respiratory distress
What are some manifestations of Hypermagnesemia?
hypomagnesemia
insufficient amount of magnesium in the extracellular fluid
less then 1.5 and can happen in alcoholics, malnutrition, and malabsorption syndromes
less then 1.5 mEq/L.
What is the lab value for hypomagnesemia?
Behavioral changes (e.g., irritability)
Increased reflexes
Muscle cramping
Involuntary muscle movements (e.g., tremors or spasms)
Tachycardia (increased heart rate)
Hypotension (low blood pressure)
what are some manifestations for hypomagnesemia?
Acid-Base Balance
equilibrium between acid and base concentrations in the body fluids; the body carefully regulates acid-base balance to maintain pH within a narrow range. Small changes in pH can significantly affect various biological processes in the body.
pH
refers to the concentration of hydrogen ions (H⁺) in a solution, which determines the acidity or alkalinity.
7.4
What is the pH level for the body (normal)?
If hydrogen ion concentration is high, pH is below 7.4.
What is considered to be an acidic pH?
above 7.4
What is considered to be basic (alkaline) pH?
Acidic
The byproducts of metabolism. these donate hydrogen ions (H⁺) to maintain pH balance.
bases (alkaline substances)
Acids are balanced by______________