Chem115 Memorization things
0.0(0)
Card Sorting
1/22
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
1
New cards
linear (2)
2 electron groups (180)
2
New cards
trigonal planar (3)
3 electron groups (120)
3
New cards
bent (3)
2 electron groups 1 lone pair (<120)
4
New cards
Tetrahedral (4)
4 electron groups (109.5)
5
New cards
trigonal pyramidal (4)
3 electron groups 1 lone pair (<109.5)
6
New cards
bent (4)
2 electron groups 2 lone pairs (<109.5)
7
New cards
octet rule exceptions
groups 2 & 3, molecules with odd number electrons, 3rd period and down
8
New cards
ion-dipole
ion attracted to dipole
9
New cards
hydrogen bonding
H atom attracted to O, N, or F
10
New cards
dipole-dipole
attractions between difference of electronegativity of atoms
11
New cards
london dispersion forces
in all molecules, temporary dipoles
12
New cards
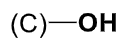
alcohol
13
New cards
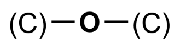
ether
14
New cards
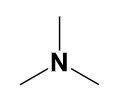
amine
15
New cards
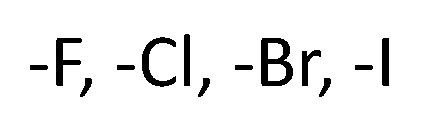
halide
16
New cards

alkene
17
New cards
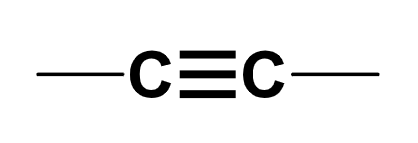
alkyne
18
New cards
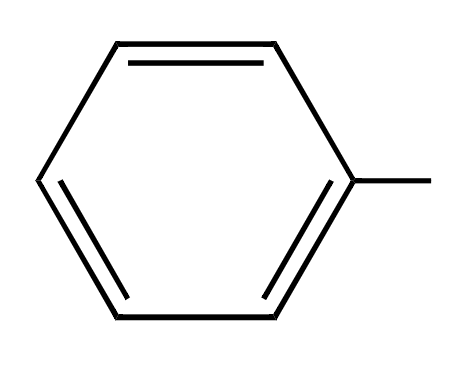
phenyl
19
New cards
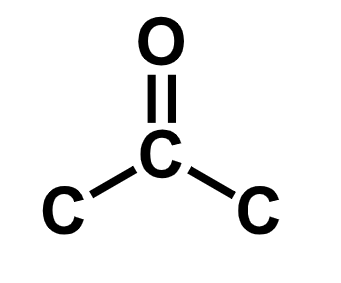
ketone
20
New cards
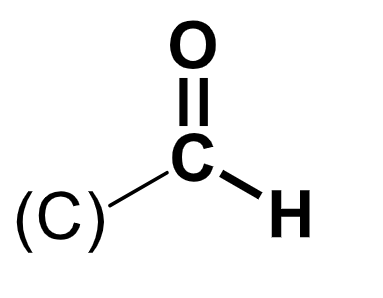
aldehyde
21
New cards
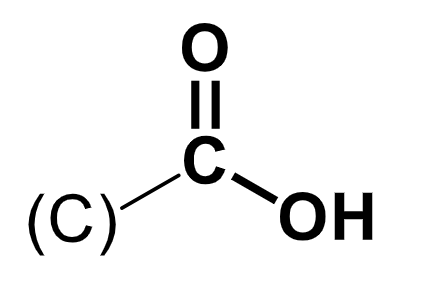
carboxylic acid
22
New cards
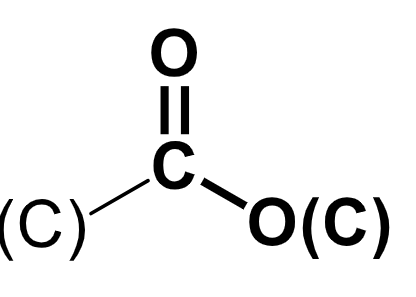
ester
23
New cards
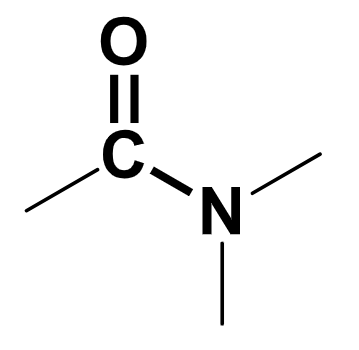
amide