Cancer Chemotherapy 1
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
considerations to chemotherapy
long-term gain vs. risk
probability of treatment success vs. quality of life
considerations of patient
type/stage of cancer
health of patient (kidney/liver function, bone marrow reserve, concurrent medical problems)
desire to undergo difficult/dangerous treatment
ability to cope with side-effects
pre-treatment screening
treatment regimen
most given in combination (synergistic, different mechanisms of action and resistance)
drugs should be given as frequently and as possible close to the maximal effective dose as possible
dosage
generally based on body surface area
pharmacokinetics, drug interactions and impact on liver, kidneys and immune system need to be considere
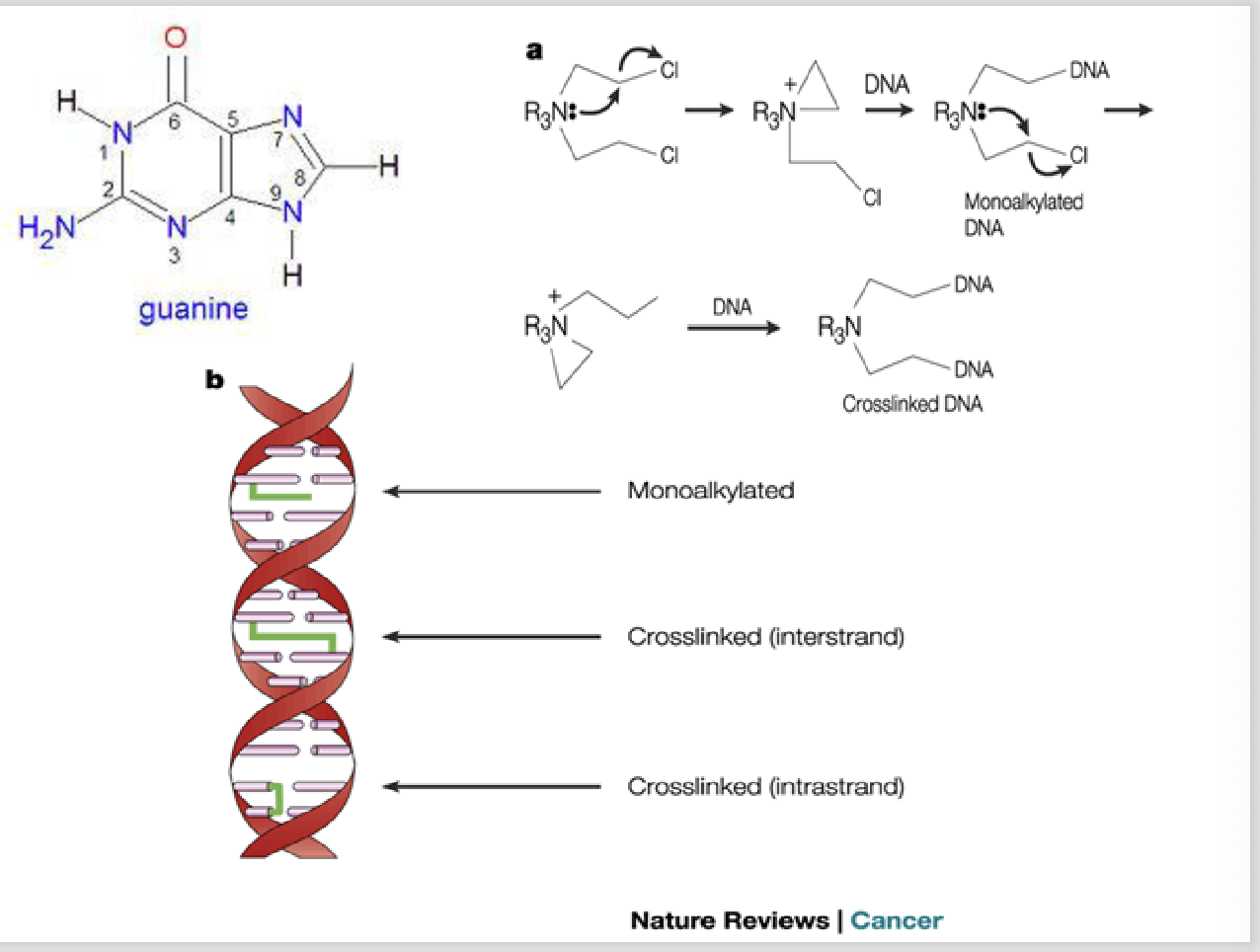
DNA alkylating agents mechanism of action
transfer alkyl group to cellular constituents
what is the major site of DNA alkylation
Major site in DNA: N7 and/or O6 of guanine
how many locations do they alkylate at
monofunctional (alkylate single DNA strand) or bifunctional (alkylate at two locations, cross-link)
what kinds of cells are more sensitive to DNA alkylation
proliferating cells more sensitive
major classes of DNA alyklators
Alkylsulfonates
methyl/ethylenimines
nitrogen mustards (clyclophosphamide)
nitrosoureas
*platinum compounds (cisplatin)
Triazenes
*technically not alkylating agents but also bind N7 and cross-link DNA
what is the most commonly used alkylating agent
cyclophosphamide
what kind of drug is a cyclophosphamide
pro-drug, administered IV or orally, activated by cytochrome P450, lipid soluble
spectrum of activity for cyclophosphamide
broad spectrum: used alone or in combination with other drugs to treat neuroblastomas, lymphomas, leukemias and colon, breast, ovarian, small cell lung and testicular cancers
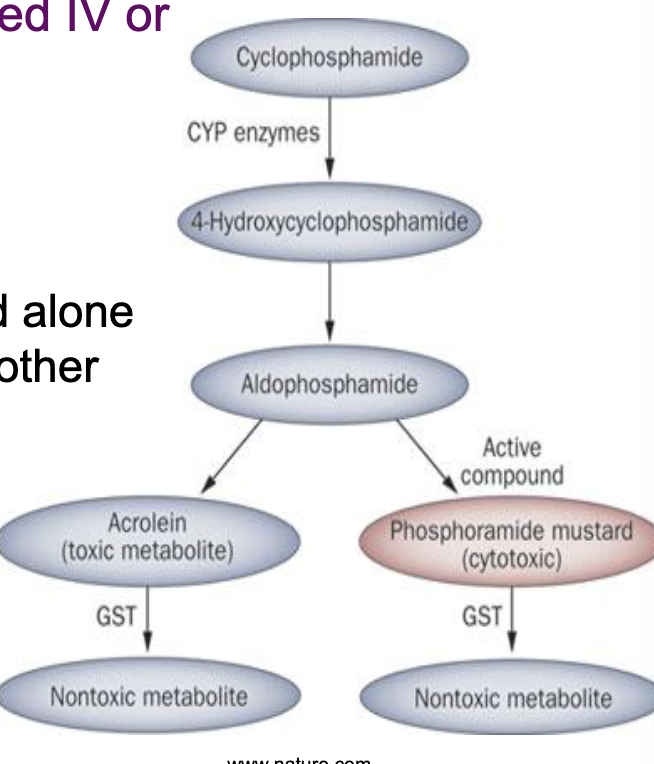
adverse effects of cyclophosphamide
less toxic than some alkylating agents due to cellular metabolism of aldophosphamide by aldehyde dehydrigenase
dose-dependent Gi disturbances, bone marrow suppression, immunosuppression, hair loss, hemorrhagic cystitis (acrolein accumulation)
increased risk of sterility, menopause, cancer
resistance mechanisms of cyclophosphamide
reaction with other cellular constituents
increased metabolism (ALDH, GST)
increased DNA repair e.g. cancer cells with high levels of O6-methylguanine-DNA methyl-transferase (MGMT) less susceptible
What is Cisplatin and what does it do
inorganic metal: covalently binds N7 and O6 of guanine also interacts with cytosine and adenine
how is cisplatin administered
IV; particularly effective for testicular, bladder and ovarian cancer.
also used to treat lymphomas, sarcomas and lung carcinomas
adverse effects of Cisplatin
bone marrow suppression
anemia
GI distress (one of most emetogenic chemotherapies)
nephrotoxicity
electrolyte imbalances
neurotoxicity (hearing loss, peripheral neuropathy)
Cisplatin resistance mechanisms
decreased access to DNA
increased DNA repairX

explain this diagram
outside the cell: Cisplatin is stable
in the bloodstream, chloride concentration is high (~100mM)
at high chloride levels, cisplatin stays in its neutral, stable form: Pt(NH3)2Cl2
entry into the cell - Cisplatin gets in two ways
a. transporter-mediated influx
transporters like CTR1 (copper transporter 1) bring cisplatin into the cell
b. passive diffusion
neutral cisplatin can also diffuse directly across the membrane
inside the cell: chloride drops → cisplatin activates
intracellular [Cl] is much lower (3-20mM)
in the low Cl environment, cisplatin undergoes hydrolysis (chloride ligands replaced by water)
activated cisplatin becomes positively charged and highly reactive (this activated form can now bind to biological molecules)
cellular fates of activated cisplatin
a. binding to DNA (therapeutic effect)
activated cisplatin travels into the nucleus
it forms DNA adducts → causes DNA damage → triggers apoptosis
alternative fate of cisplatin (resistance mechanism)
binding to detoxifying molecules
i. glutathione (GSH)
glutathione (Glu-Cys-Gly) can bind activated cisplatin
GST enzymes (glutathione-S-transferases) catalyze this
this produces cisplatin-GSH conjugates, which detoxify the drug
ii. Metallothionein
metallothionein bind metals and can sequester cisplatin, preventing it from reaching DNA
Efflux: pumping cisplatin out (contributes to resistance)
cisplatin-GSH conjugates are exported by:
a. ATP7A/ATP7B (copper transporters_
they transport cisplatin out of the cell or into vesicles
b. GS-X/MRP2 pump (multidrug resistance protein)
actively pumps cisplatin-conjugates outside the cell → excretion
this efflux decreases intracellular cisplatin → contributes to drug resistance
non-covalent DNA binding agents
antibiotics extracted from the soil microbe streptomyces that have anti-tumour activity form tight drug-DNA interactions
mechanism of action of non-covalent DNA binding agents
DNA intercalation
free radical DNA damage
DNA unwinding, impaired synthesis, single and double strand breaks
Bleomycin
forms Bleomycin-Fe(II) complex that interacts with oxygen
oxidation of complex → free radicals (O2-, OH) → DNA strand breakage & damage of other cellular constituents

how is Bleomycin administered
through variety of routes (IV, IM, SC), typically in combination with other drugs, to treat lymphomas and cervical, ovarian and testicular cancers
adverse effects of Bleomycins
pulmonary fibrosis
anaphylaxis
GI disturbances
alopecia
Resistance mechanisms of Bleomycin
Increased DNA repair
Increased drug efflux
Increased expression fo antioxidants or bleomycin hydrolase
Cyclophosphamide, methotrexate & florouracil (CMF)
commonly used regimen of breast cancer chemotherapy that combines three anti-cancer agents
regimen for CMF treatment
four-week cycle
on days 1 and 8 methotrexate and fluorouracil are given IV. Cyclophosphamide sometimes administered IV in conjunction with these drugs, or is taken as an oral tablet once a day for the first 14 days of each cycle
typical treatment involves 6-8 cycles
Antimetabolites
affect cell proliferation by interfering with DNA and RNA synthesis, thus, most effective in S-phase of cell cycle
interfere with the availability of purines and pyrimidines
major classes of antimetabolites
folate antagonists (methotrexate)
Pyrimidine analogs (5-fluorouracil)
purine analogs
sugar-modified analogs
how does methotrexate enter cell
via active transport
how does methotrexate work
folic acid inhibitor: structurally similar to folate & binds to dihydrofolate (DHF) reductase
reduces purine and pyrimidine synthesis, thus affects RNA, DNA, and protein synthesis
Methotrexate-polyglutamate metabolites retained in cells to further inhibit RNA/DNA synthesis

what is methotrexate used with
usually used in combination with other drugs for a variety of carcinomas, leukemias and lymphomas
how is methotrexate administered
orally or via injection (IV, IM, SC, IT)
resistance mechanisms of methotrexate
decreased cellular uptake
increased DHF reductase expression
decreased binding to DHF reductase
increased efflux
adverse effects of methotrexate
bone marrow suppression
GI distress, alopecia
liver damage (long term treatment)
renal damage (high dose)
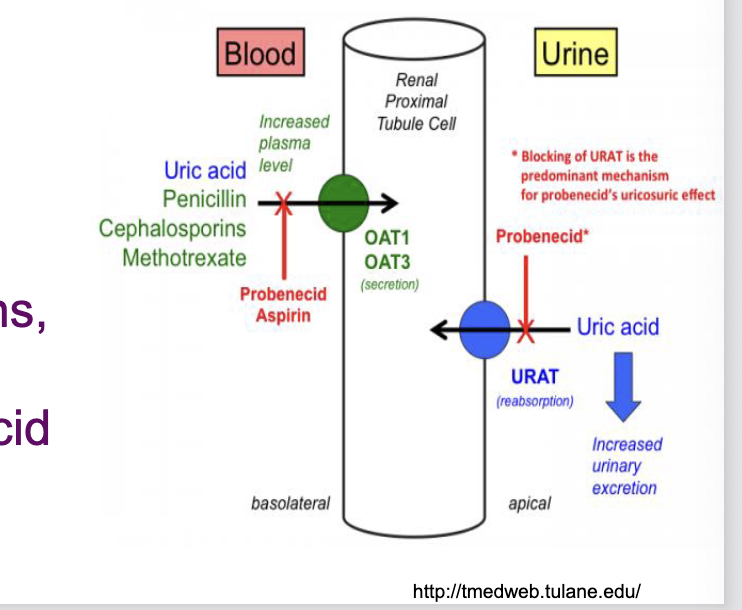
drug interactions with methotrexate
aminoglycosides decreases methotrexate absorption
NSAIDs, penicillins, cephalosporins, cisplatin, probenecid decrease methotrexate absorption
5-fluorouracil
carrier mediated transport into the cell
converted to ribosyl and deoxyrybosyl nucleotide metabolites and incorporated into RNA and DNA
inhibits thymidylate synthase (TS) → decreases thymidine synthesis → decreases DNA synthesis
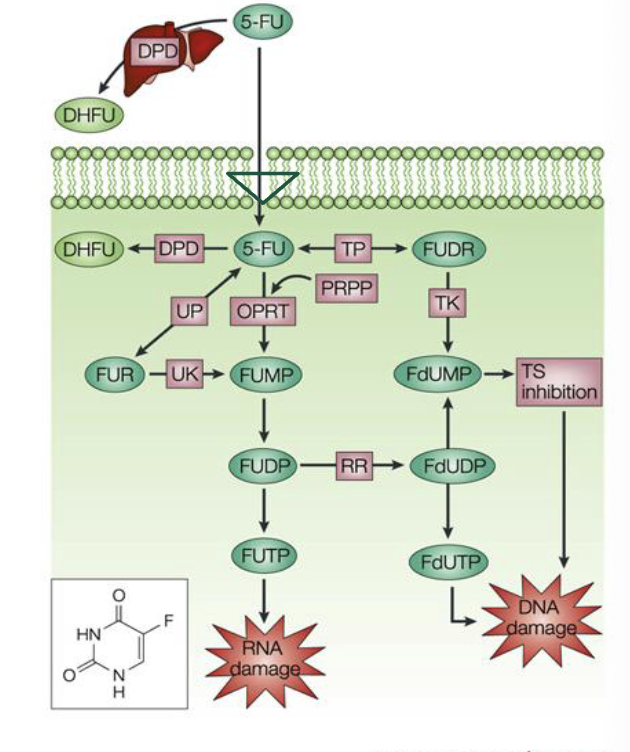
what is 5-fluorouracil used for
primarily used to treat carcinomas of breast, skin, and GI tract (esophageal, gastric, colorectal, anal)
how is 5-fluorouracil administered
IV or topically
adverse effects of 5-fluorouracil
bone marrow suppression
GI disturbances (nausea, vomiting, diarrhea, ulceration)
alopecia
cardiotoxicity (angina, arrhythmias)
skin irritation
dosing concerns of 5-fluorouracil
minimum effective dose and maximum tolerated dose very close
under and overdosing are both concerns
monitoring serum levels being investigated to maximize efficacy and minimize adverse effects
resistance mechanisms of 5-fluorouracil
decreased cell uptake
increased 5-fluorouracil metabolism
decreased conversion to nucleotide metabolite
increased thymidylate synthase activity
prolonged DNA synthesis time (DNA repair)