Pathology - Neutrophils, Granulomas, SIRS
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
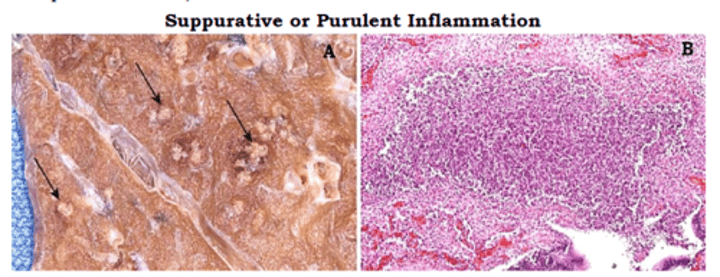
Words that also mean: neutrophilic inflammation
- Suppurative
- Purulent
- Abscess
- Pus
Timeline of neutrophilic inflammation
- Hours: Neutrophils accumulation
- Days: Pus (bacterial infection)
- Weeks: Abscess (fibrosis of pus)
What triggers a neutrophilic inflammation?
- Bacterial infection
- Immune complexes (Type III rxn)
- Trauma
- Foreign material
How is pus formed?
Bacterial infection trying to escape from neutrophilic "traps" => Pus out
Outcomes of neutrophilic inflammation
- Resolution (apoptosis of neutrophils) (if treated!)
- Chronic inflammation (if stimulus persists)
- Fibrosis
- Contained into abscess
What happens to neutrophils when infection is resolved?
Undergo apoptosis => Phagocytosed by macrophages => Disappear!
Example of morphological diagnosis of neutrophilic inflammation
Chronic severe suppurative fibrinous multifocal inflammation
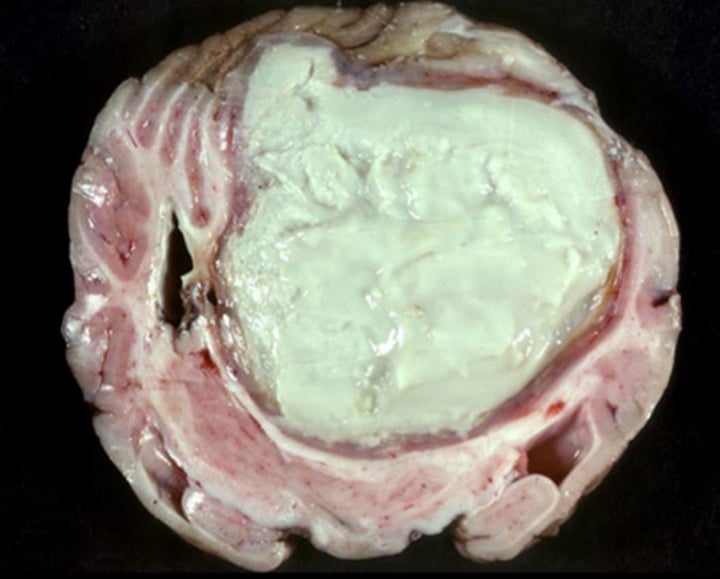
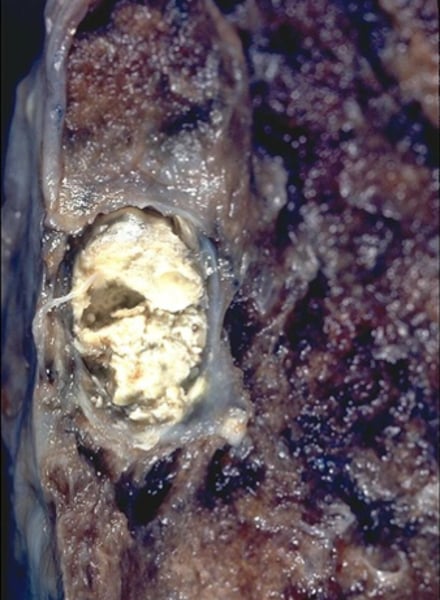
Gross appearance of neutrophilic inflammation
- Cloudy plaque
- Pus (if infected)
- Abscess (if long time fibrosis): White creamy filled nodules, tough fibrous tissues
5 steps of neutrophil response
0. Antigen recognition: Triggers cells to release mediators (PAMPs DAMPs - TLRs, Opsonization)
1. Inflammatory mediators: Stimulate vessel wall "leaks"
2. Rolling adhesion: Neutrophils roll and adhere to vessel walls via selectin
3. Firm adhesion: Integrins (neutrophil) attach to ICAM1 (vessel wall)
4. Diapedesis: Neutrophils migrate out of vessels
5. Chemotaxis: Inflammatory mediators/chemoattractants (IL8) attracts neutrophils towards injury site
What are the chemicals that help attract neutrophils? (3 names)
- Inflammatory mediators
- Chemoattractants
- Chemotaxic molecules
What would happen if an animal lacks chemical mediators (don't have CD18, IL8)?
=> Impaired neutrophil function/migration
=> Increased neutrophil levels in blood (as they can't migrate out of vessels)
=> PERSISTENT, RECURRENT INFECTIONS
Name some chemotaxic/inflammatory mediators
- Neutrophils: IL-8
- Eosinophils: Eotaxin
- Both: C5a
How do antibodies protect against infections? (4)
- Bind bacteria => Complement lysis
- Opsonisation
- Block bacteria's cell-adhesion proteins
- Neutralize toxin
3 ways for leukocytes to recognize antigens
- Opsonins coat pathogen -> attracts
- PAMPS/DAMPS - TLRs (innate) - on macrophages/leukocytes - recognize and bind to antigens => activate cells to produce inflammatory mediators
- Cytokines (mediators) trigger neutrophils action
Name some opsonins that enhance leukocytic recognition of pathogens
- IgG = Acquired (from Bcells)
- C3b = Acquired and Innate
- SP-A, MBL = Innate
How do neutrophils kill?
- Phagocytose, then kill with:
- Oxidative (ROS)
- Antimicrobials in granules
- Proteolytic enzymes in granules
- NETs = when neutrophils die -> net
Benefits and consequences of neutrophilic inflammation?
Benefits:
+ Killing bacteria (3 ways)
+ Phagocytosis -> eliminate bacteria
+ Contain infection in an area (abscess)
Consequences:
- Forming space-occupying lesion => Impair organs function/mobility, rupture organs
- ROS and proteases => Injure tissues, Pain
- Cytokines => Thrombosis, Ischemia/Infarction
- Fibrosis => toughen, stricture
- Fluid leakage (if in vessels) => Hemorrhage, Edema
Neutrophilic inflammation creating oxidative stress - how is it seen in histo?
= brown stains of malondialdehyde, that is mass produced when ROS is produced
Where are neutrophils found in healthy animals?
- Blood (vessels)
- Made from bone marrow
What trigger eosinophils?
- Parasites, worms, helminths, trematodes
- Allergy
Gross and microscopic appearance of eosinophil inflammation
- Gross: May be green
- Microscopic: Pink/eosinophilic
Explain the process of triggering eosinophils from pathogen to mediators
1. Recognition: Antigen-Ig; APC presents to lymphocytes
2. Mediators: Lymphocytes produce chemokines/mediators
3. Chemotaxis: Chemokines attract Eosinophils to site
3.2 Histamine effect: Mast cells -> histamine -> Vasodilation, hyperemia, permeability etc.
How are eosinophils different from neutrophils action?
- Similar: ROS, and proteins from granules to kill pathogen (but stronger?)
- Difference: NOT phagocytic
Generally, similar mechanisms of chemotaxis, but different mediators.
Explain type 3 hypersensitivity - how is it formed, and consequence if not resolved?
- Circulating immune complexes (of antigens + antibodies) deposit on vessel walls
- Whenever antigen meets antibody, a complex is always formed => mostly resolved (phagocytosis/complement)
-> If not resolved => cause infections via production of cytokines -> recruitment of neutrophils
=> Vasculitis: Neutrophil-induced damage
=> Platelet activation
=> Thrombosis
=> Ischemia, Infarction
=> Fluid leakage (fibrin, hemorrhage, edema)
What triggers Immune complex rxns?
- Antigens
- Neoplasia
- Drugs rxn
- Autoimmune
Sequelae of Immune complexes rxn, pathogenesis, and final consequence?
Immune complexes formed when meet antigens (ex. Strangles)
-> Trigger inflammatory mediators (cytokines, IL-8)
-> Activate complement + macrophages and neutrophils
-> Neutrophils recruitment and chemostaxis; Platelet activation
=> Vasculitis: Ischemia, infarction, leakage of fluid/edema/hemorrhage
Where do immune complexes dz usually happen and their corresponding names?
- Typically in well-vascularized tissues (glomerular, skin, synovium in joints)
- Vasculitis = skin
- Polyarthritis = joints
- Glomerular = glomerulonephritis
- Uveitis = eyes
- Systemic lupus = many tissues
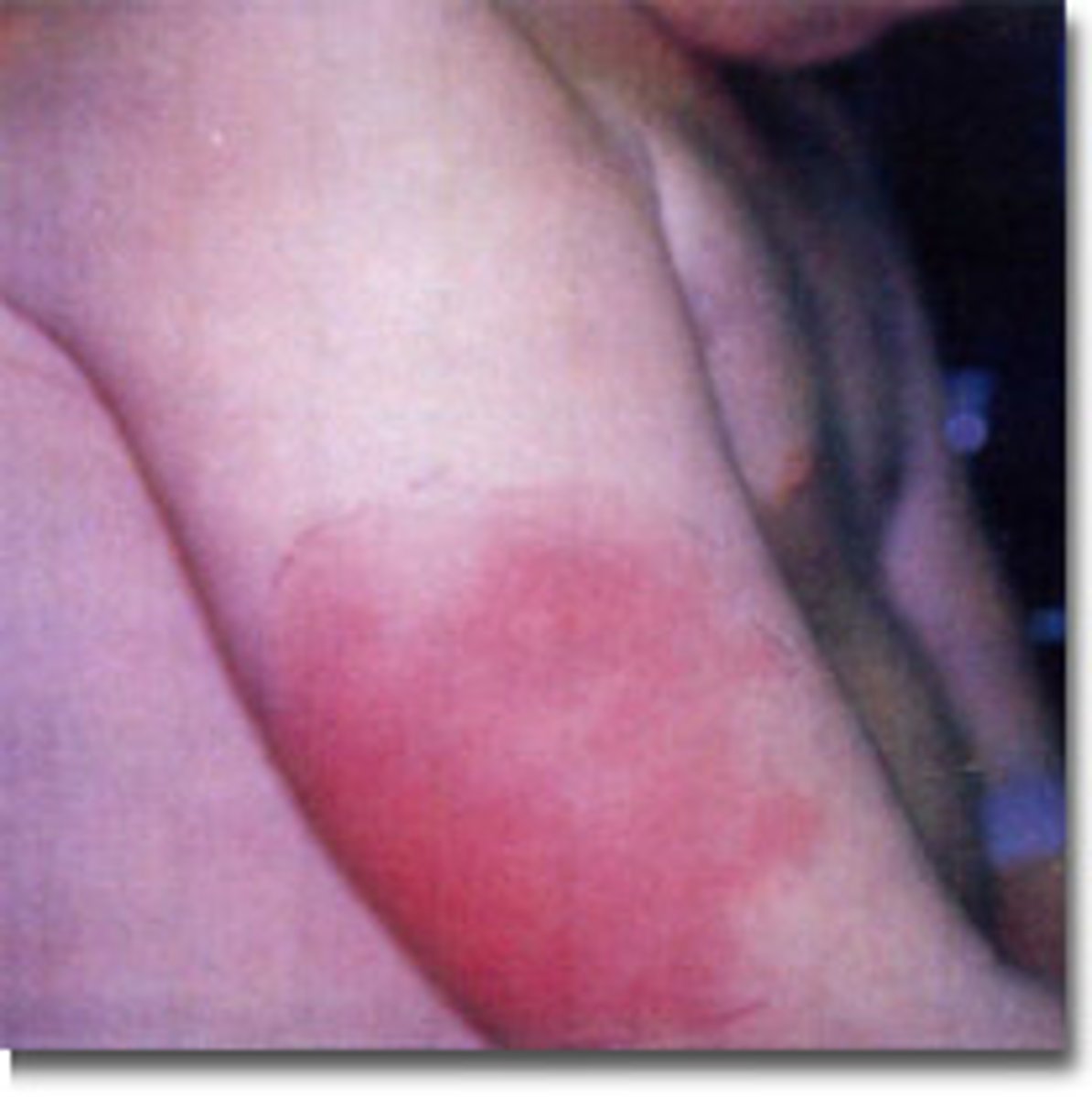
Arthus reaction
Localized Type III immune complex reaction in the skin
Bastard strangles
= Strep. equi spreading from pharynx and lymph nodes to viscera
= Septicemia, NOT immune complexes
= Bacteremia
= Distant lesion = Abcess, septic
= INFECTIOUS VASCULITIS from Strep.
Purpura hemorrhagica
Strep. equi => Immune complexes => Vasculitis
- Wound fail to heal because damage to epithelium => No mitotic cells
= NON-INFECTIOUS VASCULITIS from Strep.
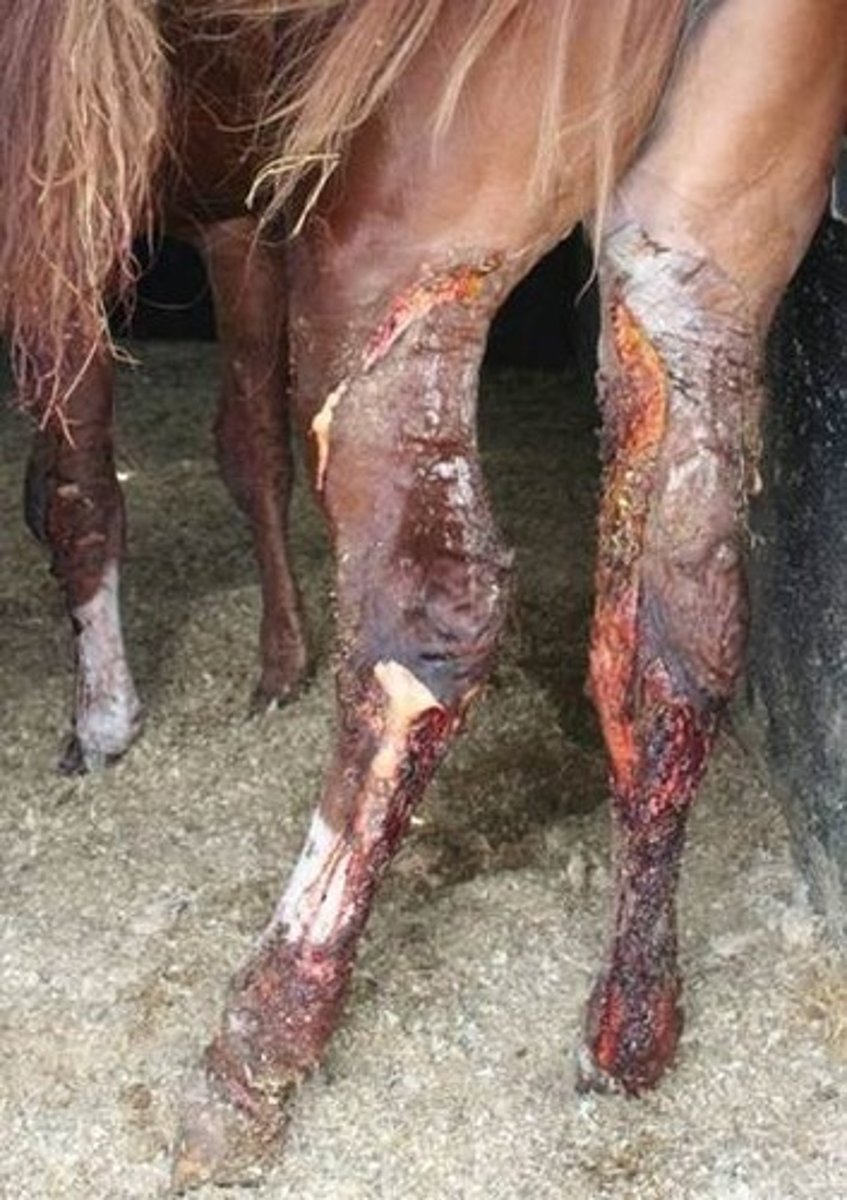
Purpura hemorrhagica vs Bastard strangles
- P. H. is non infectious, made from immune complexes
- B. S. is infectious, bacteremia
- BOTH cause vasculitis/abscess and neutrophilic inflammation
Define granulomatous inflammation
= Inflammation dominated by macrophages
Often include: fibrosis, giant & epithelioid macrophages
= Typically cell-mediated immune response (type IV)
NOT granulation tissue (this is new healing tissue!)
Define granuloma
= Nodular mass of activated macrophages (surrounded by lymphocytes rim)
= To localize pathogen to an area (similar to abscess)
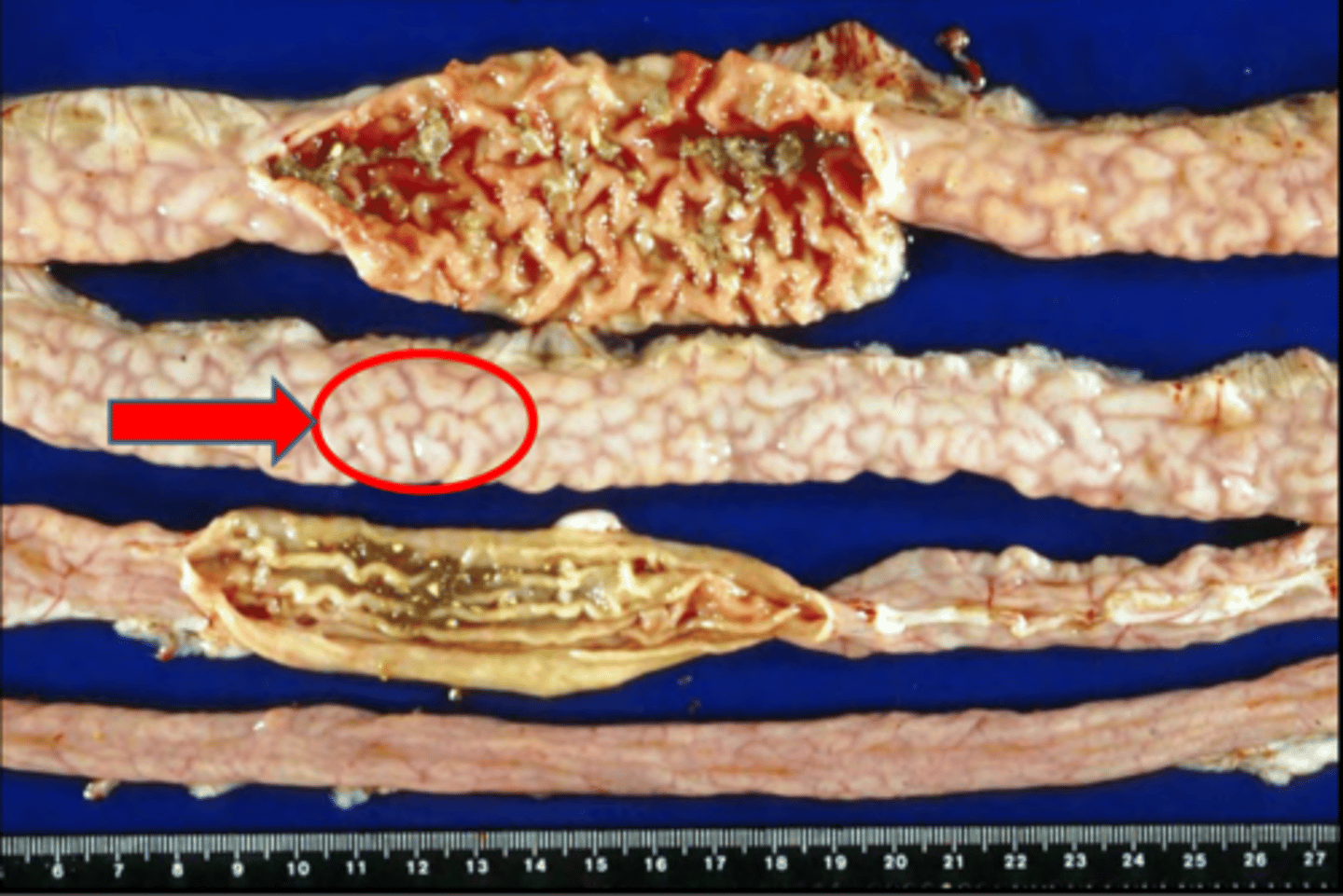
Gross and microscopic appearance of granulomatous inflammation
GROSS:
- White, tan, red
- 2 types: Homogenous, diffuse thickening, OR Central necrosis nodules
- Neoplasm-like
Ex. adenocarcinoma liver, horses' colitis
MICROSCOPIC:
- Multinucleated & Giant macrophages
- Epithelioid macrophages
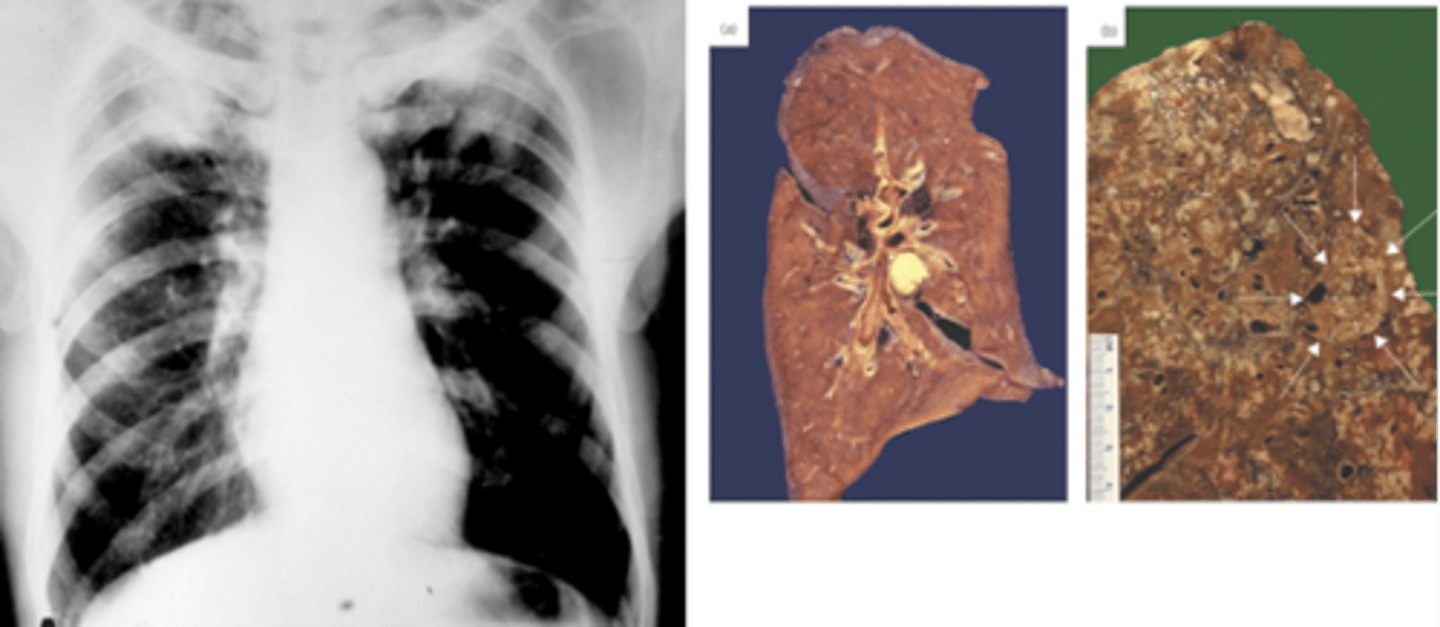
Appearance of mycobacterial infection
Nodules, necrotic centre
OR
Diffuse thickening
Pathogenesis of mycobacterial infetion inflammation (steps and pathological processes involved)
- Mycobacteria infect macrophages
-> Macrophages secrete chemokines -> Recruit more M and T-cells
-> T-cells -> IFN-y -> MORE Macrophages activation!
=> Granulomatous inflammation + Cell-mediated immunity (T-cells protection)
Define pyogranulomatous inflammation
= Inflammation involving BOTH macrophages (granulomatous) and neutrophils (pus/pyo)
Causes of granulomatous inflammation
- Foreign material
- Mycobacteria
- Fungi
- DTH type IV sensitivity reaction
Pathogenesis of granulomatous inflammation
- Response to foreign material
OR
- Immune response (type IV) w T-helpers, delayed
What's the typical time duration of granulomatous inflammation?
CHRONIC!
Sequelae of granulomatous inflammation
- Space-occupying lesion => Pressure on organs
- Cachexia: Chronic body wasting
Cachexia meaning
weakness and wasting of the body due to severe chronic illness
How are macrophages activated to kill pathogens and cause lesions?
Imagine a kid (macrophage) bringing a bag of candy (pathogen) to an adult (T-cell) to help open:
- Macrophage phagocytose antigen => Present antigen to T-helpers
- T-helpers produce IFN-y => Activate Macrophages
=> Now macrophages better kill!
- Macrophages also produce mediators (IL6, IL1) -> Permeabilty, hyperemia, vasodilation -> Edema, red, raised lesion
How do macrophages migrate to sites of injury?
SIMILAR TO NEUTROPHILS
1. Mediators
2. Rolling adhesion: Selectin
3. Firm adhesion: Integrin + ICAM1
4. Diapesis and Chemotaxis
Difference from macrophages rxn vs neutrophilic rxn?
Macrophages are present in BOTH blood and tissues!
- Blood: Monocytes
- Tissues: Macrophages
=> Rapid response!
Neutrophils MUST be recruited from blood
What do macrophages do in response to tissue injury?
- Remove necrotic debris => Repair tissues, fibrosis induced
- Kill pathogens => Defend host
- Cytokines secrete => Inflammation
- APC => Immune resp.
List some triggers of delayed-type hypersensitivity (type IV)? + a clinical example?
- Plants
- Topicals
- Textiles
- Rubber, latex
- Metal
Example: Tuberculin rxn
What's in poison ivy that triggers Type IV rxn?
Urushiol
Explain steps of type IV rxn (and relevance to granulomatous inflammation)?
- Sensitization phase (multiple exposures overtime): Allergen + Macrophages (APC) -> Present to naive T-cells (Th1) -> Form T-memory
- Elicitation phase (2-3 days after): Allergen + Macrophages (APC) -> Present to T-memory Th1 -> Secrete:
+ IFN-y: Activate macrophages => Granulomatous inflammation
+ Cytokines (IL6): Inflammation (hyperemia, permeability, vasodilation -> etc.)
+ Chemokines: Recruit MORE macrophages and lymphocytes
Explain cutaneous sterile pyogranuloma
- Dermatitis with sterile (non-infectious) granulomatous nodules
- Look like skin lumps
- Unknown cause!
= Type IV Immune-mediated WITHOUT CAUSE/pathogen
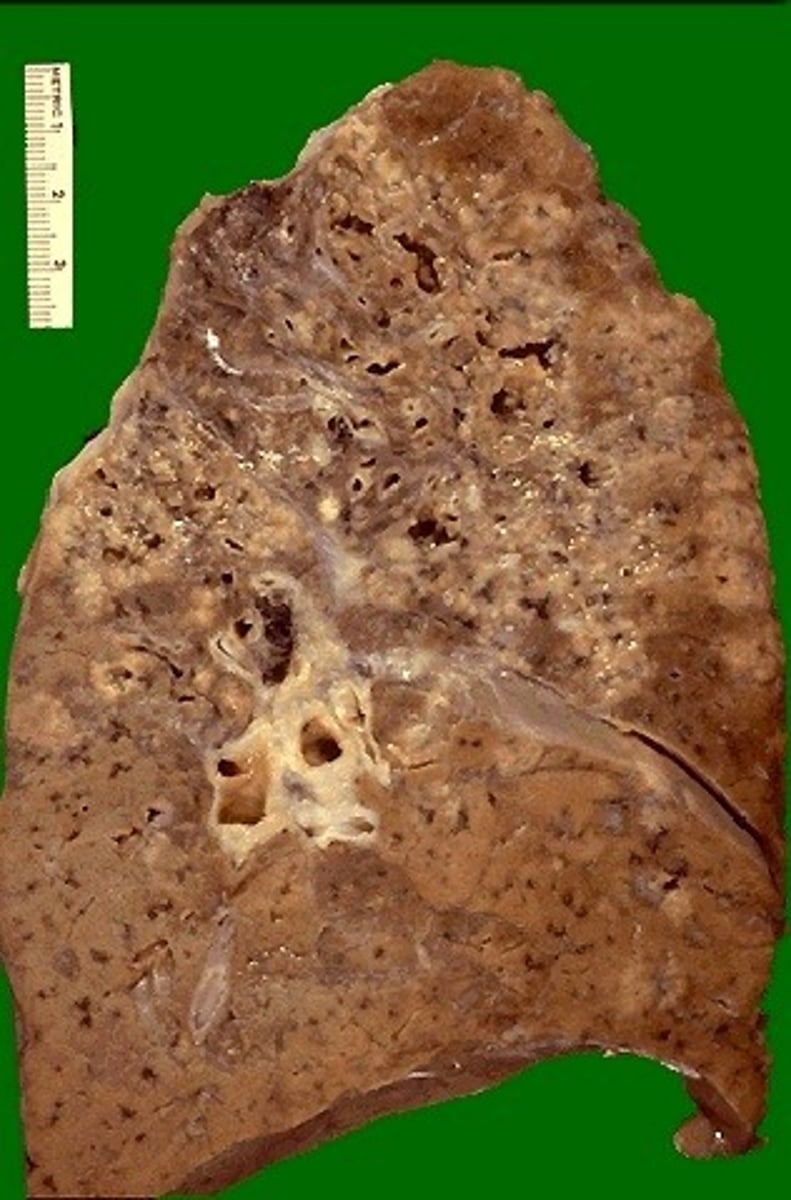
What clinical signs can a granulomatous infection in the lung cause, and how?
- Pathogen (ex. yeast) -> Macrophages and mediators
-> Histamine -> Bronchoconstriction
-> Fibrosis -> Decrease lung compliance
-> Vasodilation/Hyperemia -> Swelling, redness -> Decrease gas exchange
How to diagnose whether it is other types of rxn, or delayed (type IV)?
- Re-expose patient to allergen
=> Quick rnx = Other types (I, II, III)
=> Delayed after 2+ days = Delayed IV
What types of cells do you expect to see/react for each type of these pathogens?
Parasites
Mycobacteria
Bacteria
Virus, protozoa
Parasites = Eosinophils
Mycobacteria = Macrophages
Bacteria = Neutrophils
Virus, protozoa = Lymphocytes/T-cytotoxic
What can be the cause of lesions with each of these types of cells?
Eosinophils
Macrophages
Neutrophils
Lymphocytes/T-cytotoxic
Eosinophils = Parasites, Type I rxn
Macrophages = Mycobacteria, Fungi, Type IV rxn, foreign
Neutrophils = Bacteria, Type II rxn, foreign
Lymphocytes/T-cytotoxic = Virus, protozoa
Systemic Inflammatory Response Syndrome (SIRS) meaning
= Systemic consequences of either:
- LOCALIZED inflammation (ex. pneumonia, pancreatitis, leukocytosis, cytokinemia) + SYSTEMIC distribution of inflammatory mediators/cytokine storm
OR
- SYSTEMIC inflammation (anaphylaxis, liver injury, bacteremia)
SIRS vs Acute Phase Response
SAME THING!
Acute phase response = Systemic ADAPTIVE (Acute = Adaptive) response to severe inflammation
Sepsis meaning
= SIRS caused by infection
Bacteremia meaning
= Bacteria in systemic circulation (w or w/o dz)
Explain pathogenesis and steps of SIRS
- DAMPS and PAMPS + PRRS
-> Mediators (IL6, TNF-a, Complement cascade) causing:
- Acute response: Fever, depress, anorexia, leukocytosis, liver production of acute phase proteins
- Vasodilation: Systemic hypotension -> Ischemia! (ex. liver zone 3 necrosis and injury)
- Permeability: Edema
- Leuko activation: ROS, proteases
=> Tissue damage and systemic inflammation
- DAMPS and PAMPS + PRRS also activates endothelium: Increase tissue factors, decreases protein C and thrombomodulin
-> Coagulation -> Disseminated intravascular coagulation (DIC)
=> Consumptive Coagulopathy & Hemorrhage
Septicemia meaning
= Bacteremia WITH CLINICAL DZ
Explain some body changes/responses in Acute Phase Response and what organs it's caused by
Mostly physiologic:
- Fever - Hypothalamus
- Anorexia, depression - Brain
- Leukocytosis - BM
- Increase acute phase protein synthesis in liver and plasma levels (ex. C protein, fibrinogen) - Liver
- Cachexia
- Cortisol (stress)
Definition, benefits and consequence of of acute phase proteins
Definition: Proteins (serum) that increased in response to inflammation
Benefits:
- Control pathogens
- Stop inflammation
- Minimize damage in tissues
Consequence: Albumin secretion
Explain steps and pathogenesis of SIRS
- Inflammatory stimulus + macrophages
-> Mediators release (IL-6, IL-1, TNF-a) -> travel to other organs via blood (short half-lives; those with longer half-lives are inhibited at times)
-> Induce other factors (PGE2, CSFs)
-> Symptoms (fever, anorexic, leukocytose, acute phase proteins)
What are CSFs?
colony stimulating factors
What are acute phase proteins and what are they used for?
= Proteins made during acute phase response, which is a systemic cytokine effect
= Diagnose systemic inflammation
+ Ruminant: Fibrinogen, haptoglobins
+ Dogs/cats: Serum amyloid A
Autoimmune disease meaning
= Failure of self tolerance
= Ig or T-cell mediated
Explain steps to autoimmune rxn
- Igs or T-cells/T-cytotoxic react to self-antigens
-> Activate complement + opsonization
-> Inflammation and Cell lysis
Explain 3 mechanisms of autoimmune dz damages (which cell mediates?) + example dz (2 each)
- Ig-mediated complement and opsonization (Type 1 & 2): Hemolytic anemia, Thrombocytopenia,
- Immune complex + macrophage (Type 3): Vasculitis, uveitis
- T-cell mediated (Type 4): Lupus, hypothyroid
Examples of autoimmune dz
- Hemolytic anemia
- Thrombocytopenia
- Pemphigus
- Hypothyroidism
- Hypoadrenocortism
- Systemic lupus
General benefits of inflammation
- Contain injury to a site
- Remove necrotic tissues
- Initiate tissue repair(sometimes)
- Eliminate/kill pathogen
Gelatinous transformation of fat - meaning
- Bone marrow has gelatinous materials instead of fat
- Serous atrophy
= Chronic resorption of fat, only protein remains
How to diagnose SIRS or general inflammation?
- Fever
- Leukocytosis
- Acute phase protein levels high
Which organs are damaged in SIRS and consequences?
- Kidney
- Liver: Increased acute phase proteins
- Lung
- Heart
- Intestine
- Brain/hypothalamus: anorexia, depression, fever
- BM: leukocytosis
What's the major reason of death from SIRS?
- NOT from primary damage
- From secondary damages to organs