Esters and Carboxylic acid
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Carboxylic acid
They occur widely in nature, often combined with alcohols or other functional groups, as in fats, oils, and waxes.
Carboxylic acid
They are components of many foods, medicines, and household products.
Carboxylic acidd
Not surprisingly, many of them are best known by common names based on Latin and Greek words that describe their source.
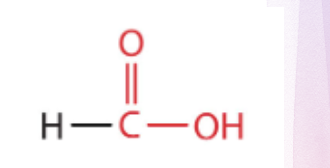
Formic acid
Acetic acid
Propionic acid
Butyric acid
These carboxylic acid devicatives (?) are miscible in water
Oxidation: w/ catalyst of Potassion dichromate
The formation of carboxylic acid
Oxidation formation of carbocylic acid
What reaction?

Bicarbonate test
Litmus test
Ester test for carboxylic acid
Identification test of carbxylic acid
Ester
have the general formula RCOOR′
Ester
where R may be a hydrogen atom, an alkyl group, or an aryl group, and R′ may be an alkyl group or an aryl group but not a hydrogen atom.
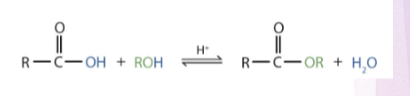
Esterification
Preparation of ester
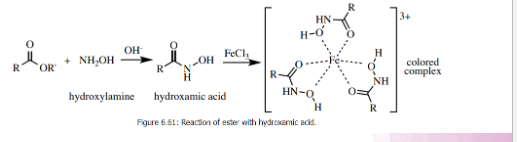
Ferric hydroxamate test
Test for Ester
Ferric hydroxamate test
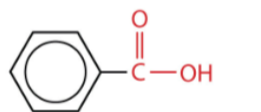
What is evident structure in the photo?
Esterification
What is this reaction?
Formic acid
what is the structure?
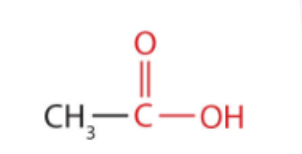
Acetic acid
What is the structure?
Benzoic acid
What is this structure?