Chapter 3: Globular Proteins
1/106
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
107 Terms
Hemeproteins
group of specialized proteins that contain heme as a tightly bound prosthetic group
role is dictated by the environment created by the three dimensional
structure of the protein
heme group of a cytochrome
functions as an electron carrier that is alternately oxidized and reduced
heme group of the enzyme catalase
is part of the active site of the enzyme that catalyzes the breakdown of hydrogen peroxide
hemoglobin and myoglobin
the two most abundant hemeproteins in humans
the heme group serves to reversibly bind oxygen (O2)
Heme
a complex of protoporphyrin IX and ferrous iron (Fe2+)
iron
held in the center of the heme molecule by bonds to the four nitrogens of the porphyrin ring
heme Fe2+
can form two additional bonds, one on each side of the planar porphyrin ring
myoglobin and hemoglobin
In ___, one of these positions is coordinated to the side chain of a histidine residue of the globin molecule, whereas the other position is available to bind O2
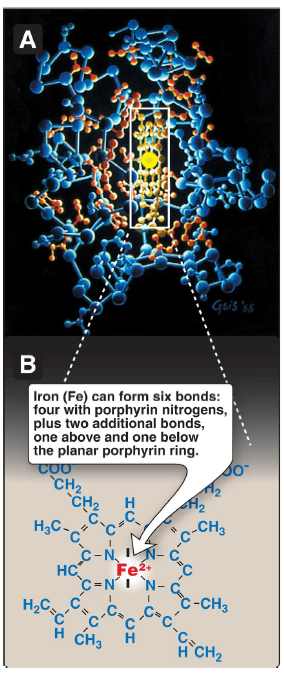
Iron (Fe)
can form six bonds: four with porphyrin nitrogens, plus two additional bonds, one above and one below the planar porphyrin ring
Myoglobin
a hemeprotein present in heart and skeletal muscle
functions both as an oxygen reservoir and as an oxygen carrier that increases the rate of oxygen transport within the muscle cell
consists of a single polypeptide chain that is structurally similar to the individual polypeptide chains of the tetrameric hemoglobin molecule
mouse myoglobin double knockouts
have an apparently normal phenotype
Myoglobin consists of a single polypeptide chain that is structurally similar to the individual polypeptide chains of the tetrameric hemoglobin molecule.
This homology makes myoglobin a useful model for interpreting some of the more complex properties of hemoglobin.
Myoglobin
is a compact molecule, with ~80% of its polypeptide chain folded into eight stretches of α-helix
presence of proline, whose five-membered ring cannot be accommodated in an α-helix
β-bends and loops stabilized by hydrogen bonds and ionic bonds
How are the a-helical regions terminated?
electrostatic interactions or salt bridges
Ionic bonds are also termed ___
nonpolar amino acids
The interior of the globular myoglobin molecule is composed almost entirely of ___
They are packed closely together, forming a structure
stabilized by hydrophobic interactions between these clustered residues
polar amino acids
located almost exclusively on the surface, where they can form hydrogen bonds, both with each other and with water
crevice
The heme group of the myoglobin molecule sits in a ___, which is lined with nonpolar amino acids. Notable exceptions are two histidine residues
proximal histidine (F8)
One, the ___, binds directly to the Fe2+ of heme
distal histidine (E7)
The second, or ___, does not directly interact with the heme group but helps stabilize the binding of O2 to Fe2+.
the protein, or globin, portion of myoglobin
creates a special microenvironment for the heme that permits the reversible binding of one oxygen molecule (oxygenation).
TRUE
[TRUE OR FALSE] The simultaneous loss of electrons by Fe2+ (oxidation to the ferric [Fe3+] form) occurs only rarely.
Hemoglobin
found exclusively in red blood cells (RBC), where its main function is to transport O2 from the lungs to the capillaries of the tissues
Hemoglobin A
the major hemoglobin in adults, is composed of four polypeptide chains (two α chains and two β chains) held together by noncovalent interactions
each chain (subunit) has stretches of α-helical structure and a hydrophobic heme-binding pocket similar to that described for myoglobin
tetrameric hemoglobin molecule
is structurally and functionally more complex than myoglobin
hemoglobin
can transport protons (H+) and carbon dioxide (CO2) from the tissues to the lungs and can carry four molecules of O2 from the lungs to the cells of the body
allosteric effectors
the oxygen-binding properties of hemoglobin are regulated by interaction with ___
TRUE
[TRUE OR FALSE] Obtaining O2 from the atmosphere solely by diffusion greatly limits the size of organisms. Circulatory systems overcome this, but transport molecules such as hemoglobin are also required because O2 is only slightly soluble in aqueous solutions such as blood.
Quaternary structure
The hemoglobin tetramer can be envisioned as composed of two identical dimers, (αβ)1 and (αβ)2.
The two polypeptide chains within each dimer are held tightly together primarily by hydrophobic interactions.
Hydrophobic amino acid residues are localized not only in the interior of the molecule but also in a region on the surface of each subunit. Multiple interchain hydrophobic interactions form strong associations between α-subunits and β-subunits in the dimers.
The two dimers are held together primarily by polar bonds,
The weaker interactions between the dimers allow them to move with respect to one other.
This movement results in the two dimers occupying different relative positions in deoxyhemoglobin as compared with oxyhemoglobin
weak ionic and hydrogen bonds
occur between two αβ dimers in the deoxygenated state
strong interactions, primarily hydrophobic, between α and β chains
form stable αβ dimers
broken
some ionic and hydrogen bonds between two αβ dimers are ___ in the oxygenated state
T or taut
structure of deoxyhemoglobin
R or relaxed
structure of oxyhemoglobin
“T,” or taut (tense) form
deoxy form of hemoglobin
the two αβ dimers interact through a network of ionic bonds and hydrogen bonds that constrain the movement of the polypeptide chains
T conformation
low-oxygen-affinity form of hemoglobin
R form
The binding of O2 to hemoglobin causes the rupture of some of the polar bonds between the two αβ dimers, allowing movement.
Specifically, the binding of O2 to the heme Fe2+ pulls the iron into the
plane of the heme.
Because the iron is also linked to the proximal histidine (F8), the resulting movement of the globin chains alters the interface between the αβ dimers.
R conformation
the high-oxygen-affinity form of hemoglobin
Myoglobin
can bind only one molecule of O2, because it contains only one heme group
Hemoglobin
can bind four molecules of O2, one at each of its four heme groups
TRUE
[TRUE OR FALSE] The degree of saturation (Y) of these oxygen binding sites on all myoglobin or hemoglobin molecules can vary between zero (all sites are empty) and 100% (all sites are full)
Pulse oximetry
a noninvasive, indirect method of measuring the oxygen saturation of arterial blood based on differences in light absorption by oxyhemoglobin and deoxyhemoglobin
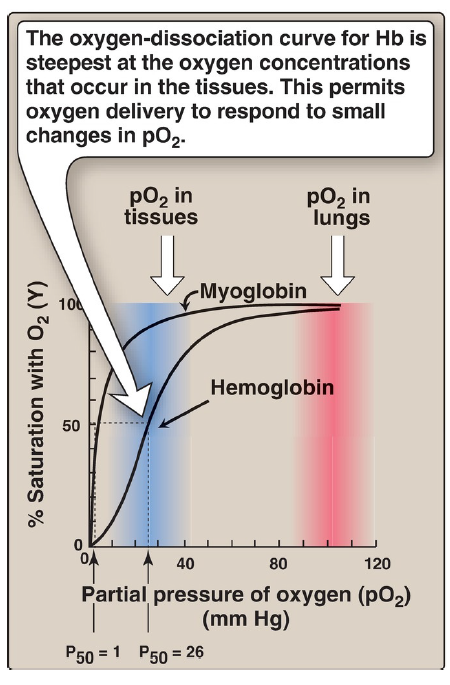
oxygen-dissociation curve for Hb
is the steepest at the oxygen concentrations that occur in the tissues
this permits oxygen delivery to respond to small changes in pO2
oxygen-dissociation curve
A plot of Y measured at different partial pressures of oxygen (pO2) is called the ___
The curves for myoglobin and hemoglobin show important differences
This graph illustrates that myoglobin has a higher oxygen affinity at all pO2 values than does hemoglobin
~1 mm Hg
The partial pressure of oxygen needed to achieve half saturation of the binding sites (P50) is ___ for myoglobin
26 mm Hg
The partial pressure of oxygen needed to achieve half saturation of the binding sites (P50) is for hemoglobin
TRUE
[TRUE OR FALSE] The higher the oxygen affinity (that is, the more tightly O2 binds), the lower the P50
hyperbolic
The oxygen-dissociation curve for myoglobin has a ___ shape.
This reflects the fact that myoglobin reversibly binds a single molecule of O2.
oxygenated (MbO2) and deoxygenated (Mb) myoglobin exist in a simple equilibrium:
The equilibrium is shifted to the right or to the left as O2 is added to or removed from the system. [Note: Myoglobin is designed to bind O2 released by hemoglobin at the low pO2 found in muscle. Myoglobin, in turn, releases O2 within the muscle cell in response to oxygen demand.]
![<ul><li><p>The oxygen-dissociation curve for myoglobin has a ___ shape.</p></li><li><p>This reflects the fact that myoglobin reversibly binds a single molecule of O<sub>2</sub>.</p></li><li><p>oxygenated (MbO<sub>2</sub>) and deoxygenated (Mb) myoglobin exist in a simple equilibrium:</p></li><li><p>The equilibrium is shifted to the right or to the left as O2 is added to or removed from the system. [Note: Myoglobin is designed to bind O2 released by hemoglobin at the low pO2 found in muscle. Myoglobin, in turn, releases O2 within the muscle cell in response to oxygen demand.]</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/69d1d7f8-4ca7-465b-81e8-70a63e71a7eb.png)
sigmoidal
The oxygen-dissociation curve for hemoglobin is ___ in shape, indicating that the subunits cooperate in binding O2.
Cooperative binding of O2 by the four subunits of hemoglobin means that the binding of an oxygen molecule at one subunit increases the oxygen affinity of the remaining subunits in the same hemoglobin tetramer (Fig. 3.7). Although it is more difficult for the first oxygen molecule to bind to hemoglobin, the subsequent binding of oxygen molecules occurs with high affinity, as shown by the steep upward curve in the region near 20–30 mm Hg.
increasing
Hemoglobin (Hb) binds successive molecules of oxygen (O2) with __ affinity.
pO2
pH of the environment
partial pressure of carbon dioxide (pCO2)
availability of 2,3-bisphosphoglycerate (2,3-BPG)
collectively called allosteric (“other site”) effectors, because their interaction at one site on the tetrameric hemoglobin molecule causes structural changes that affect the binding of O2 to the heme iron at other sites on the molecule
binding of O2 to monomeric myoglobin
not influenced by allosteric effectors
Oxygen
The sigmoidal oxygen-dissociation curve reflects specific structural changes that are initiated at one subunit and transmitted to other subunits in the hemoglobin tetramer. The net effect of this cooperativity is that the affinity of hemoglobin for the last oxygen molecule bound is ~300 times greater than its affinity for the first oxygen molecule bound. Oxygen, then, is an allosteric effector of hemoglobin. It stabilizes the R form.
Loading and unloading oxygen
The cooperative binding of O2 allows hemoglobin to deliver more O2 to the tissues in response to relatively small changes in the pO2. This can be seen in Figure 3.6, which indicates pO2 in the alveoli of the lung and the capillaries of the tissues. For example, in the lung, oxygen concentration is high, and hemoglobin becomes virtually saturated (or “loaded”) with O2. In contrast, in the peripheral tissues, oxyhemoglobin releases (or “unloads”) much of its O2 for use in the oxidative metabolism of the tissues.
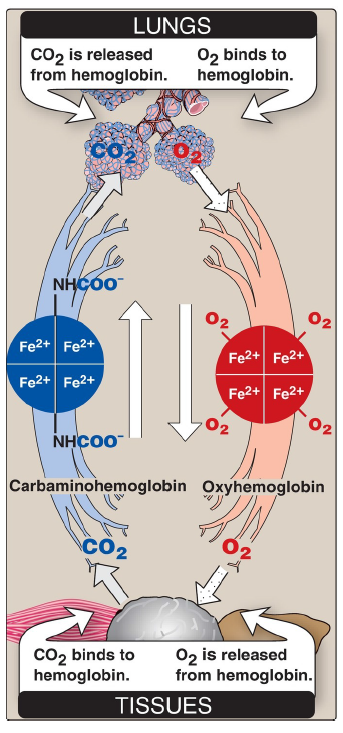
CO2
released from hemoglobin in the lungs
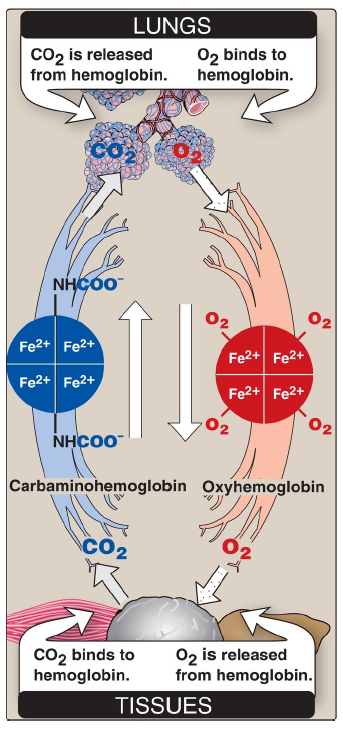
O2
binds to hemoglobin in the lungs
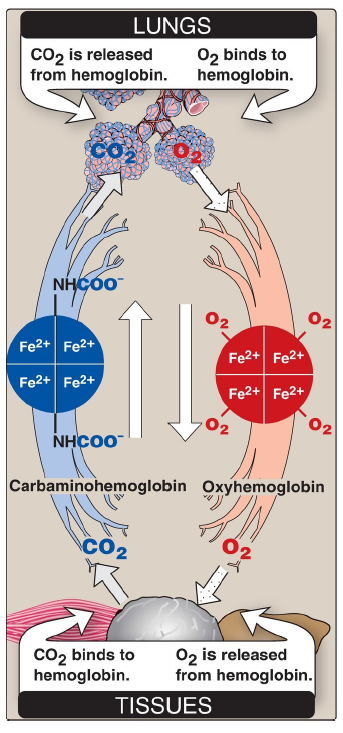
CO2
binds to hemoglobin in the tissues
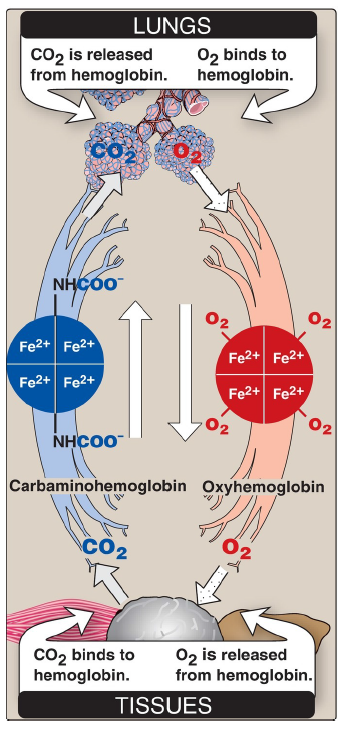
O2
is released from hemoglobin in the tissues
Significance of the sigmoidal oxygen-dissociation curve
The steep slope of the oxygen-dissociation curve over the range of oxygen concentrations that occur between the lungs and the tissues permits hemoglobin to carry and deliver O2 efficiently from sites of high to sites of low pO2. A molecule with a hyperbolic oxygen-dissociation curve, such as myoglobin, could not achieve the same degree of O2 release within this range of pO2. Instead, it would have maximum affinity for O2 throughout this oxygen pressure range and, therefore, would deliver no O2 to the tissues.
Bohr effect
The release of O2 from hemoglobin is enhanced when the pH is lowered (proton concentration [H+] is increased) or when the hemoglobin is in the presence of an increased pCO2. Both result in decreased oxygen affinity of hemoglobin and, therefore, a shift to the right in the oxygen-dissociation curve (Fig. 3.9). Both, then, stabilize the T (deoxy) form. This change in oxygen binding is called the ___. Conversely, raising the pH or lowering the concentration of CO2 results in a greater oxygen affinity, a shift to the left in the oxygen-dissociation curve, and stabilization of the R (oxy) form.
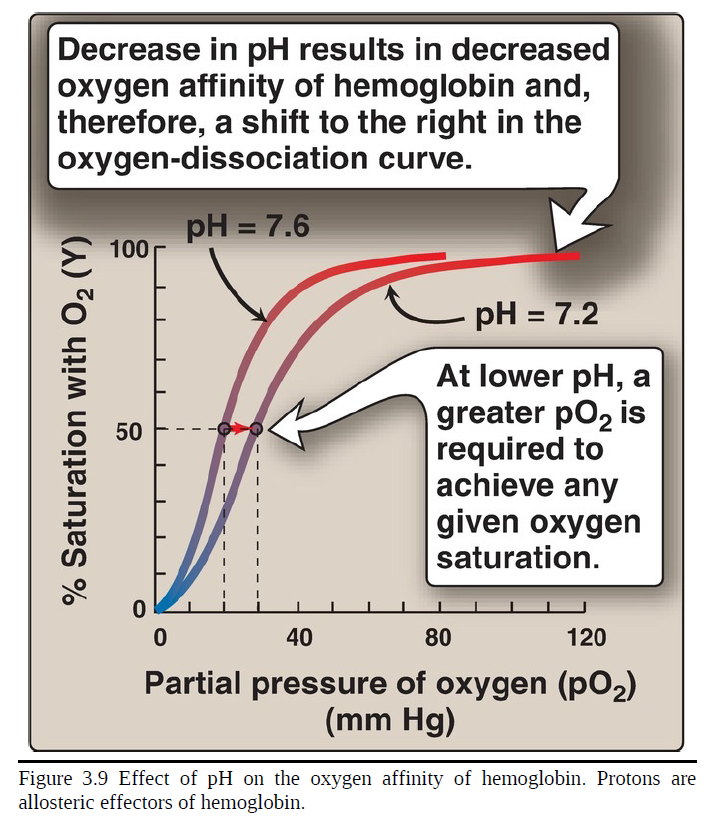
decrease
___ in pH results in decreased oxygen affinity of hemoglobin and, therefore, a shift to the right in the oxygen-dissociation curve
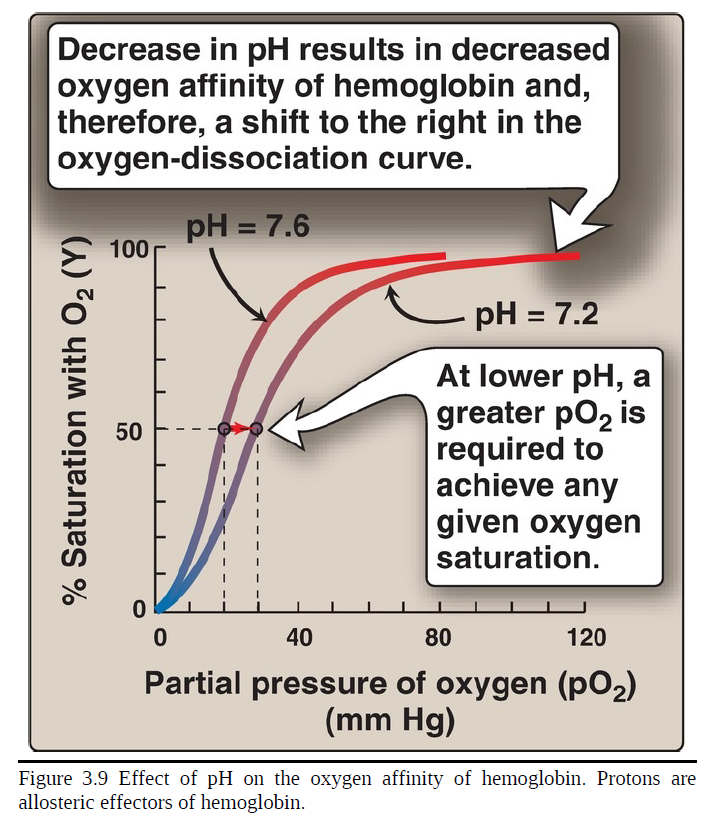
greater
at lower pH, a ___ pO2 is required to achieve any given oxygen saturation
carbonic anhydrase
Source of the protons that lower pH: The concentration of both H+ and
CO2 in the capillaries of metabolically active tissues is higher than that
observed in alveolar capillaries of the lungs, where CO2 is released
into the expired air. In the tissues, CO2 is converted by zinc-containing ___ to carbonic acid
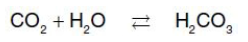
bicarbonate
the major blood buffer
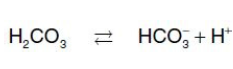
TRUE
[TRUE OR FALSE] The H+ produced by this pair of reactions contributes to the lowering of pH. This differential pH gradient (that is, lungs having a higher pH and tissues a lower pH) favors the unloading of O2 in the peripheral tissues and the loading of O2 in the lung. Thus, the oxygen affinity of the hemoglobin molecule responds to small shifts in pH between the lungs and oxygen-consuming tissues, making hemoglobin a more efficient transporter of O2.
Mechanism of the Bohr effect
The Bohr effect reflects the fact that the deoxy form of hemoglobin has a greater affinity for H+ than does oxyhemoglobin. This is caused by ionizable groups such as specific histidine side chains that have a higher pKa in deoxyhemoglobin than in oxyhemoglobin. Therefore, an increase in the concentration of H+ (resulting in a decrease in pH) causes these groups to become protonated (charged) and able to form ionic bonds (salt bridges). These bonds preferentially stabilize the deoxy form of hemoglobin, producing a decrease in oxygen affinity. [Note: Hemoglobin, then, is an important blood buffer.]
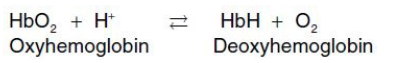
where an increase in H+ (or a lower pO2) shifts the equilibrium to the right (favoring deoxyhemoglobin), whereas an increase in pO2 (or a decrease in H+) shifts the equilibrium to the left.
The Bohr effect can be represented schematically as:
2,3-BPG
an important regulator of the binding of O2 to hemoglobin
the most abundant organic phosphate in the RBC, where its concentration is approximately that of hemoglobin
is synthesized from an intermediate of the glycolytic pathway
TRUE
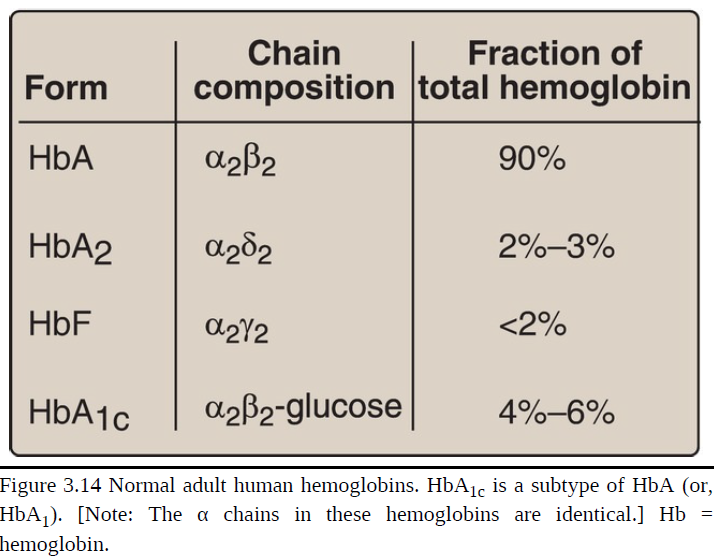
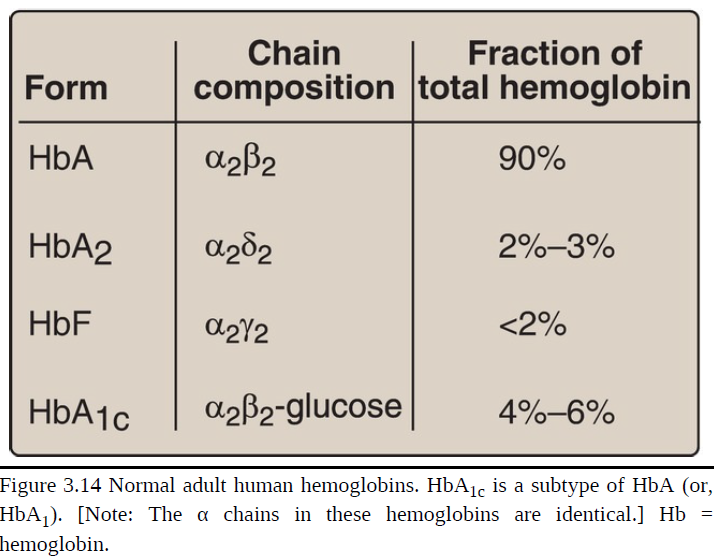
[TRUE OR FALSE] It is important to remember that human hemoglobin A (HbA) is just one member of a functionally and structurally related family of proteins, the hemoglobins (Fig. 3.14). Each of these oxygen-carrying proteins is a tetramer, composed of two α-globin (or α-like) polypeptides and two β-globin (or β-like) polypeptides. Certain hemoglobins, such as HbF, are normally synthesized only during fetal development, whereas others, such as HbA2, are synthesized in the adult, although at low levels compared with HbA. HbA can also become modified by the covalent addition of a hexose
Fetal hemoglobin
HbF is a tetramer consisting of two α chains identical to those found in HbA, plus two γ chains (α2γ2; see Fig. 3.14). The γ chains are members of the β-globin gene family.
TRUE
[TRUE OR FALSE] HbF synthesis during development: In the first month after conception, embryonic hemoglobins such as Hb Gower 1, composed of two α-like zeta (ζ) chains and two β-like epsilon (ε) chains (ζ2ε2), are synthesized by the embryonic yolk sac. In the fifth week of gestation, the site of globin synthesis shifts, first to the liver and then to the marrow, and the primary product is HbF. HbF is the major hemoglobin found in the fetus and newborn, accounting for ~60% of the total hemoglobin in the RBC during the last months of fetal life (Fig. 3.15). HbA synthesis starts in the bone marrow at about the eighth month of pregnancy and gradually replaces HbF. Figure 3.15 shows the relative production of each type of hemoglobin chain during fetal and postnatal life. [Note: HbF represents <2% of the hemoglobin in most adults and is concentrated in RBC known as F cells.]
TRUE
[TRUE OR FALSE] 2,3-BPG binding to HbF: Under physiologic conditions, HbF has a higher oxygen affinity than does HbA as a result of HbF only weakly binding 2,3-BPG. [Note: The γ-globin chains of HbF lack some of the positively charged amino acids that are responsible for binding 2,3-BPG in the β-globin chains.] Because 2,3-BPG serves to reduce the oxygen affinity of hemoglobin, the weaker interaction between 2,3-BPG and HbF results in a higher oxygen affinity for HbF relative to HbA. In contrast, if both HbA and HbF are stripped of their 2,3-BPG, they then have a similar oxygen affinity. The higher oxygen affinity of HbF facilitates the transfer of O2 from the maternal circulation across the placenta to the RBC of the fetus.
Hemoglobin A2 (HbA2)
a minor component of normal adult hemoglobin, first appearing shortly before birth and, ultimately, constituting ~2% of the total hemoglobin
composed of two α-globin chains and two δ-globin chains (α2δ2)
Hemoglobin A1c (HbA1c)
Under physiological conditions, it is slowly glycated (nonenzymically condensed with a hexose), the extent of glycation being dependent on the plasma concentration of the hexose.
The most abundant form of glycated hemoglobin
Has glucose residues attached predominantly to the amino groups of the N-terminal valines of the β-globin chains.
Increased amounts are found in RBC of patients with diabetes mellitus, because their HbA has contact with higher glucose concentrations during the 120-day lifetime of these cells.
TRUE
[TRUE OR FALSE] To understand diseases resulting from genetic alterations in the structure or synthesis of hemoglobin, it is necessary to grasp how the hemoglobin genes, which direct the synthesis of the different globin chains, are structurally organized into gene families and also how they are expressed.
α-globin and β-globin subunits
The genes coding for the ___ of the hemoglobin chains occur in two separate gene clusters (or families) located on two different chromosomes
α-gene cluster on chromosome 16
contains two genes for the α-globin chains
contains the ζ gene that is expressed early in development as an α-globin-like component of embryonic hemoglobin
Globin gene families
contain globin-like genes that are not expressed, that is, their genetic information is not used to produce globin chains
These are called pseudogenes.
chromosome 11
A single gene for the β-globin chain is located on ___
ε gene
two γ genes
δ gene
There are an additional four β-globin-like genes:
ε geneε gene
like the ζ gene, is expressed early in embryonic development
two γ genes
Gγ and Aγ that are expressed in HbF
TRUE
[TRUE OR FALSE] Expression of a globin gene begins in the nucleus of RBC precursors, where the DNA sequence encoding the gene is transcribed. The ribonucleic acid (RNA) produced by transcription is actually a precursor of the messenger RNA (mRNA) that is used as a template for the synthesis of a globin chain. Before it can serve this function, two noncoding stretches of RNA (introns) must be removed from the mRNA precursor sequence and the remaining three fragments (exons) joined in a linear manner. The resulting mature mRNA enters the cytosol, where its genetic information is translated, producing a globin chain.
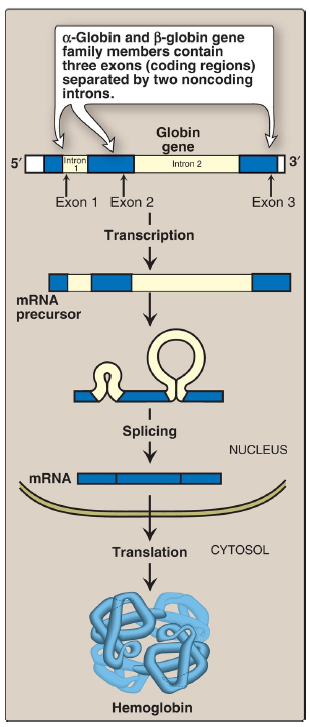
α-globin and β-globin gene family members
contain three exons (coding regions) separated by the two noncoding introns
Hemoglobinopathies
defined as a group of genetic disorders caused by production of a structurally abnormal hemoglobin molecule, synthesis of insufficient quantities of normal hemoglobin, or, rarely, both
sickle cell anemia (HbS)
hemoglobin C disease (HbC)
hemoglobin SC disease (HbS + HbC = HbSC)
thalassemias
are representative hemoglobinopathies that can have severe clinical consequences
sickle cell anemia (HbS)
hemoglobin C disease (HbC)
hemoglobin SC disease (HbS + HbC = HbSC)
result from production of hemoglobin with an altered amino acid sequence (qualitative hemoglobinopathy)
thalassemias
caused by decreased
production of normal hemoglobin (quantitative hemoglobinopathy)
Sickle cell anemia (hemoglobin S disease)
most common of the RBC sickling diseases
genetic disorder caused by a single nucleotide substitution (a point
mutation) in the gene for β-globin
It is the most common inherited blood disorder in the United States, affecting 50,000 Americans.
occurs primarily in the African American population, affecting 1 in 500
newborn African American infants
an autosomal recessive disorder
occurs in individuals who have inherited two mutant genes (one from each parent) that code for synthesis of the β chains of the globin molecules
The mutant β-globin chain is designated βS, and the resulting hemoglobin, α2βS2, is referred to as HbS.
An infant does not begin showing symptoms of the disease until sufficient HbF has been replaced by HbS so that sickling can occur.
Characterized by lifelong episodes of pain (“crises”), chronic hemolytic
anemia with associated hyperbilirubinemia, and increased
susceptibility to infections, usually beginning in infancy.
Other symptoms include acute chest syndrome, stroke, splenic and renal dysfunction, and bone changes due to marrow hyperplasia. Life expectancy is reduced. Heterozygotes, representing 1 in 12 African Americans, have one normal and one sickle cell gene. The blood cells of such heterozygotes contain both HbS and HbA, and these individuals have sickle cell trait. They usually do not show clinical symptoms (but may under conditions of extreme physical exertion with dehydration) and can have a normal life span.
<20 days
The lifetime of a RBC in sickle cell anemia is ___, compared with 120 days for normal RBC, hence, the anemia.
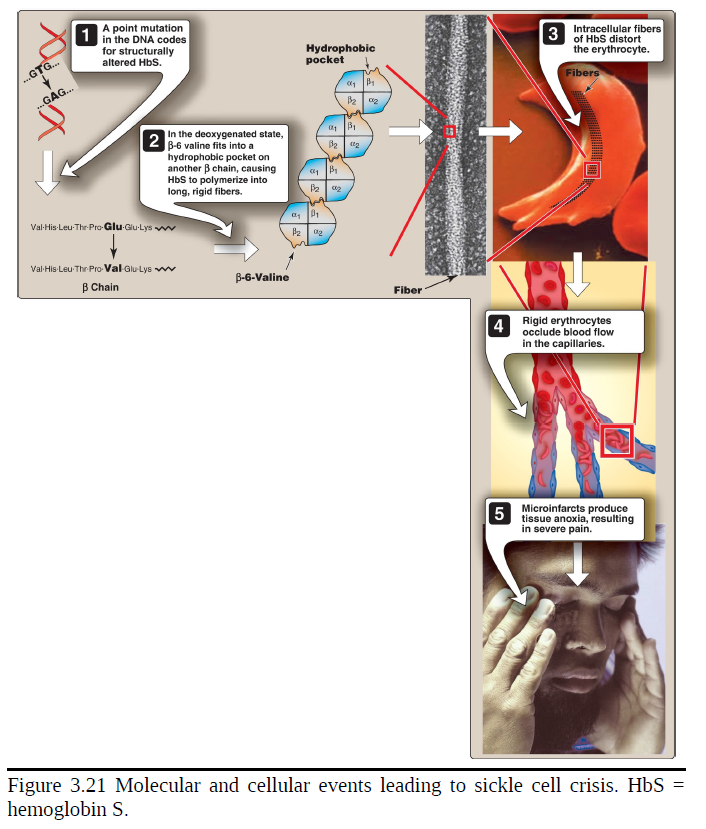
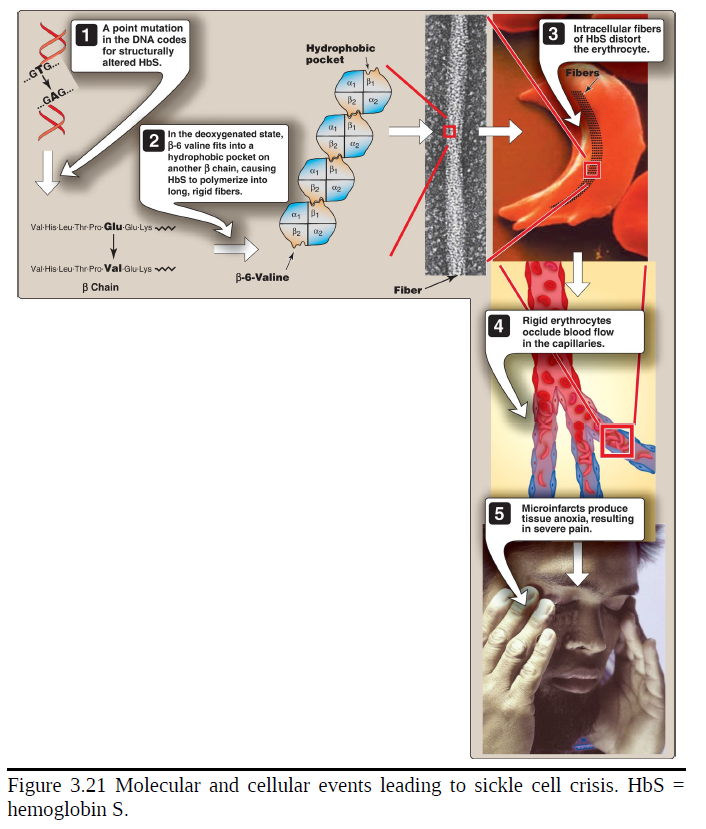
Amino acid substitution in HbS β chains
A molecule of HbS contains two normal α-globin chains and two mutant β-globin chains (βS), in which glutamate at position six has been replaced with valine. Therefore, during electrophoresis at alkaline pH, HbS migrates more slowly toward the anode (positive electrode) than does HbA. This altered mobility of HbS is a result of the absence of the negatively charged glutamate residues in the two β chains, thereby rendering HbS less negative than HbA. [Note: Electrophoresis of hemoglobin obtained from lysed RBC is routinely used in the diagnosis of sickle cell trait and sickle cell anemia (or, sickle cell disease). DNA analysis also is used.
Hemoglobins
are negatively charged and migrate toward the anode
Sickling and tissue anoxia
The replacement of the charged glutamate with the nonpolar valine forms a protrusion on the β chain that fits into a complementary site on the β chain of another hemoglobin molecule in the cell. At low oxygen tension, deoxyhemoglobin S polymerizes inside the RBC, forming a network of insoluble fibrous polymers that stiffen and distort the cell, producing rigid, misshapen RBC. Such sickled cells frequently block the flow of blood in the narrow capillaries. This interruption in the supply of O2 leads to localized anoxia (oxygen deprivation) in the tissue, causing pain and eventually ischemic death (infarction) of cells in the vicinity of the blockage. The anoxia also leads to an increase in deoxygenated HbS. [Note: The mean diameter of RBC is 7.5 μm, whereas that of the microvasculature is 3–4 μm. Compared to normal RBC, sickled cells have a decreased ability to deform and an increased tendency to adhere to vessel walls. This makes moving through small vessels difficult, thereby causing microvascular occlusion.]
Variables that increase sickling
The extent of sickling and, therefore, the severity of disease are enhanced by any variable that increases the proportion of HbS in the deoxy state (that is, reduces the oxygen affinity of HbS). These variables include decreased pO2, increased pCO2, decreased pH, dehydration, and an increased concentration of 2,3-BPG in RBC.
Treatment
Therapy involves adequate hydration, analgesics, aggressive antibiotic therapy if infection is present, and transfusions in patients at high risk for fatal occlusion of blood vessels. Intermittent transfusions with packed RBC reduce the risk of stroke, but the benefits must be weighed against the complications of transfusion, which include iron overload that can result in hemosiderosis, bloodborne infections, and immunologic complications. Hydroxyurea (hydroxycarbamide), an antitumor drug, is therapeutically useful because it increases circulating levels of HbF, which decreases RBC sickling. This leads to decreased frequency of painful crises and reduces mortality. Stem cell transplantation is possible. [Note: The morbidity and mortality associated with sickle cell anemia has led to its inclusion in newborn screening panels to allow prophylactic antibiotic therapy to begin soon after the birth of an affected child.]
Possible selective advantage of the heterozygous state
The high frequency of the βS mutation among black Africans, despite its damaging effects in the homozygous state, suggests that a selective advantage exists for heterozygous individuals. For example, heterozygotes for the sickle cell gene are less susceptible to the severe malaria caused by the parasite Plasmodium falciparum. This organism spends an obligatory part of its life cycle in the RBC. One theory is that because these cells in individuals heterozygous for HbS, like those in homozygotes, have a shorter life span than normal, the parasite cannot complete the intracellular stage of its development. This may provide a selective advantage to heterozygotes living in regions where malaria is a major cause of death. For example, in Africa, the geographic distribution of sickle cell anemia is similar to that of malaria.
Hemoglobin C disease
Like HbS, HbC is a hemoglobin variant that has a single amino acid substitution in the sixth position of the β-globin chain (see Fig. 3.19). In HbC, however, a lysine is substituted for the glutamate (as compared with a valine substitution in HbS). [Note: This substitution causes HbC to move more slowly toward the anode than HbA or HbS does (see Fig. 3.20).] Rare patients homozygous for HbC generally have a relatively mild, chronic hemolytic anemia. They do not suffer from infarctive crises, and no specific therapy is required.