4.1 Introduction to Alkanes
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
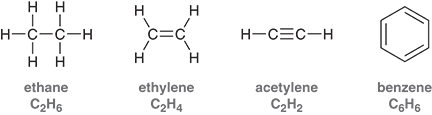
What are the hydrocarbons?
compounds that only contain carbons and hydrogens
Ex: ethane, ethylene, acetylene, benzene
2
New cards
What are saturated hydrocarbons?
Hydrocarbons that only consist of carbon and hydrogen atoms with a single bond
They are also called alkanes
usually end in the suffix “ane”
Ex: Ethane → has no pi bonds

3
New cards
What will we focus on in this chapter?
How to name alkanes
4
New cards
What is nomenclature?
It is the procedure for naming chemical compounds