2.2-Biological Molecules (copy)
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
109 Terms
what groups can biological molecules be placed in?
- carbohydrates
- protein
- lipids
- nucleic acids
- (water)
what are carbohydrates used for?
- slow releasing energy
- structure (plants)
what are proteins used for?
- growth and repair
- enzymes
- hormones
- structure
- antibodies
what are lipids used for?
- insulation
- energy
- hormones
- protection
- nerve cells
what are nucleic acids used for?
genetic information
\
what is water used for?
- support
- solvent
- transport
how many covalent bonds does carbon form?
4
how many covalent bonds does nitrogen form
3
how many covalent bonds does oxygen form
2
how many covalent bonds does hydrogen form
1
hydroxyl group
-OH
carboxyl group
-COOH
amine group
-NH₂
variable group
-R
Types of bond formed between biological molecules
Covalent bonds- electrons shared
Ionic bonds- electrons transferred
Hydrogen bonds- unequal sharing of electrons= molecules are polar, bonds are weak but provide strength together
what is a monomer
a single molecule
what is a polymer
lots of monomers joined together
condensation reactions vs hydrolysis
Hydrolysis
H2O used
covalent bonds broken down
molecules get smaller
Condensation reaction
H2O molecules released
new covalent bond formed
makes bigger molecules
Hydrogen bonding in water
1 oxygen atom is covalently bonded to 2 hydrogen atoms
sharing of electrons between hydrogen and oxygen is uneven as oxygen has more protons
oxygen- weak negatively charged region
hydrogen- weak positively charged region
polarity causes hydrogen bonds to form between positive and negative regions of adjacent water molecules
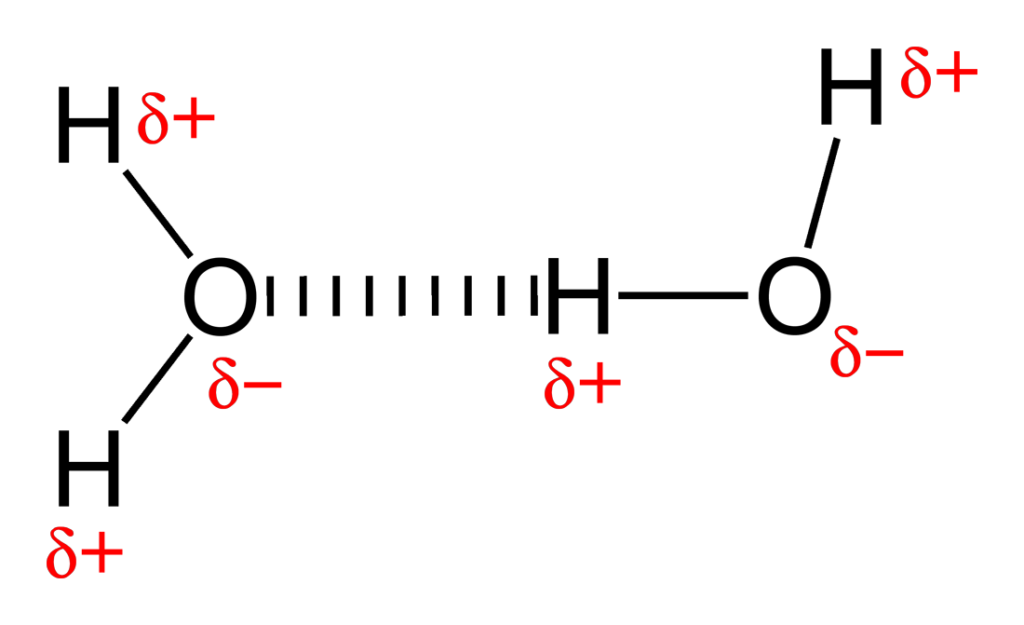
properties of water
liquid
density
solvent
cohesion and surface tension
high specific heat capacity
high latent heat of vaporisation
(reactant)
why is water a liquid
hydrogen bonds= difficult for water molecules to escape and turn into a gas
very polar, so liquid
low viscosity despite hydrogen bonds
how is waters liquidity useful in biology
provides a habitat e.g. rivers, lakes
form major components of tissues in organisms
reaction medium for chemical reactions
effective transport medium e.g. blood
density in water
normally density increases as liquid cools
water’s density increases until 4°C
between 4°C and freezing point, the molecules align themselves in a structure less dense than liquid water due to water’s polarity
how is water’s density useful
provides a stable environment for aquatic organisms to live through winter
ponds and other bodies of water insulated against extreme cold
layer of ice reduce rate of heat loss from rest of pond.
water as a solvent
polarity of water means that oppositely charged ions are attracted
water molecules gather around charged areas and separate them
particles dissolve and form a solution
molecules and ions can move around and react
how are water’s solvent properties useful
allows chemical reactions to take place in cells- dissolved solutes are more chemically reactive when they are free to move around
metabolites can be transported efficiently
cohesion and surface tension in water
cohesion →hydrogen bonds between water molecules pull them together → doesn’t spread out
water molecules on the surface are hydrogen bonded to the ones below them
more attracted to water molecules than air above them→ molecules pulled inwards so more resistance on surface=surface tension
uses of water’s cohesion and surface tension
columns of water in plant vascular tissue are pulled up the xylem tissue together from roots
insects e.g. pond skaters can walk on water
water’s high specific heat capacity
hydrogen bonds= lots of heat energy required to increase Ek and temp of water → high shc
doesn’t heat up/cool down quickly
uses of water’s high shc
living things require stable temperatures for enzyme controlled reactions to happen properly
aquatic life needs stable environment in which to live
high latent heat of vapourisation
molecules break away from each other to become a gas when water evaporates
large amount of energy needed for water molecules to evaporate
uses of water’s high latent heat of vapourisation
helps cool things down and keep temp stable
uses of carbohydrates
energy store
provide energy
structural units
form larger molecules
general formula for carbohydrates
Cn(H2O)n
simple carbohydrate (subsections and examples)
sugars-small, sweet tasting, soluble
Monosaccharides→ glucose, fructose, galactose
Disaccharides→ sucrose, maltose, lactose
polysaccharides and examples
large, non-sweet, insoluble
e.g. starch, glycogen, cellulose
how are monosaccharides grouped (+general group names)
grouped by number of carbon atoms:
3→ triose
5→ pentose
6→ hexose
alpha (α) glucose
-OH group below
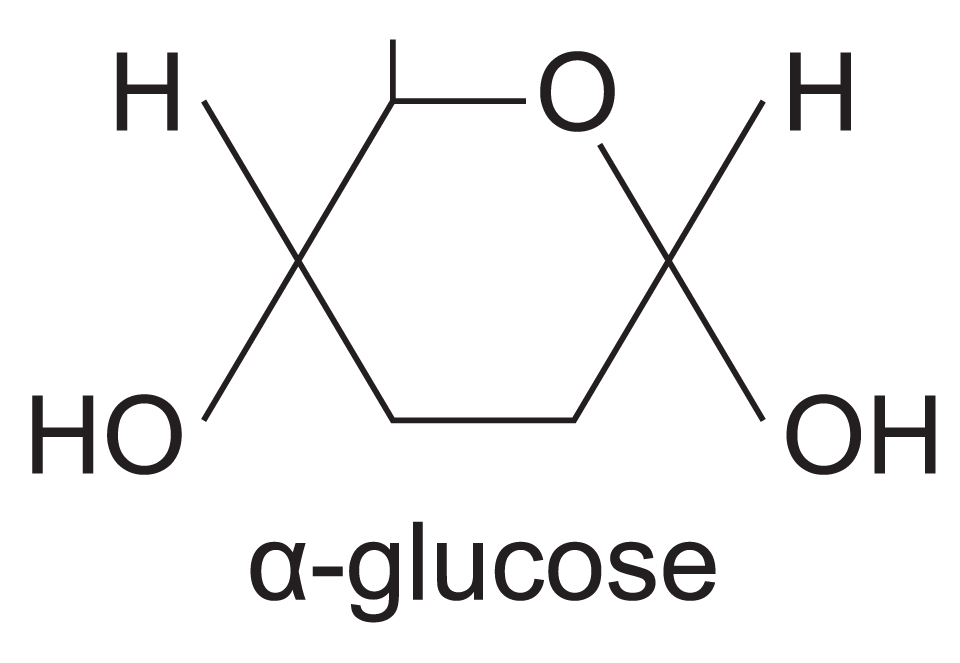
beta (β) glucose
-OH group above
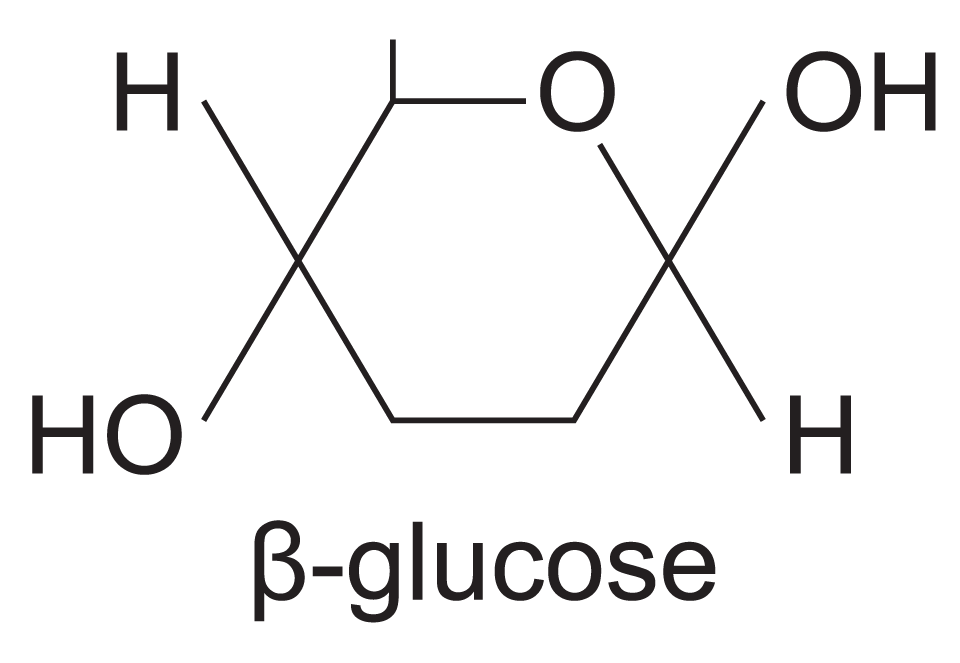
other hexose sugars
fructose- sweeter than glucose, main sugar found in fruit and nectar, very soluble
galactose- not as soluble as glucose, has important role in production of glycoprotein and glycolipids
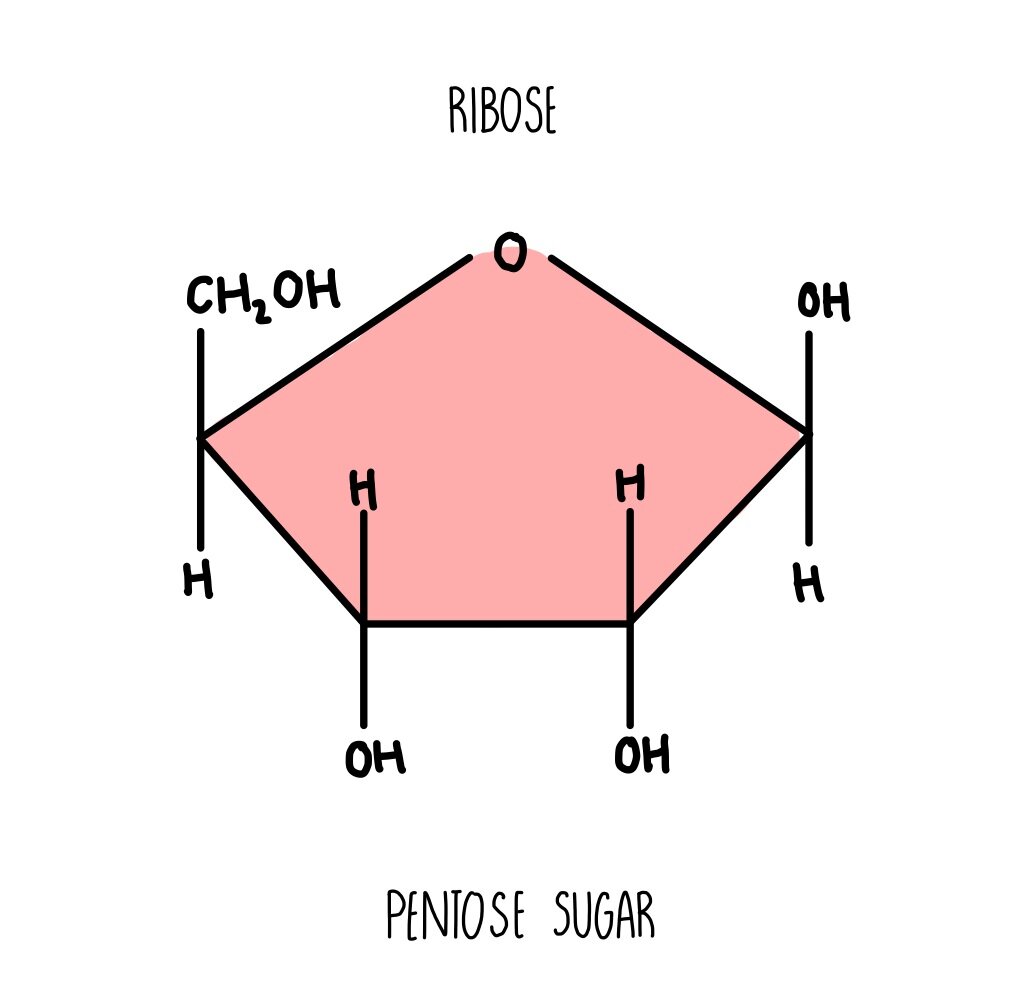
ribose
-OH attached to carbon 2
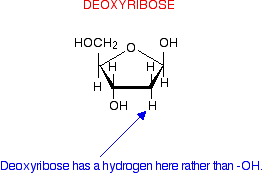
deoxyribose
-no OH attached to carbon 2
what is a reducing sugar
can donate electrons (oxidises carbonyl group)
detected using Benedict’s test
reduces copper sulfate into copper oxide
what is a non-reducing sugar
cannot donate electrons
must be hydrolysed to break disaccharide into 2 monosaccharides
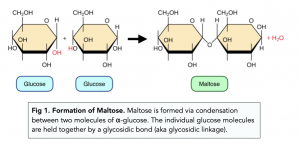
what is a disaccharide
formed from 2 monosaccharides joined together by a glycosidic bond during a condensation reaction
what monosaccharides make maltose
α-glucose+α-glucose
what monosaccharides make sucrose
α-glucose+fructose
what monosaccharides makes lactose
α-glucose+galactose
making disaccharides (e.g. maltose)
C6H12O6+C6H12O6 → C12 H22 O11 +H2O
homopolysaccharide
polymer made of identical sugar molecules
heteropolysaccharide
polymer made of more than one type of monosaccharide
what is glucose stored as
glycogen
amylose
amylopectin
what monomer forms glycogen and starch
alpha glucose
structure of amylose
unbranched
1,4 glycosidic bonds
coils into helix- stabilised by hydrogen bonds
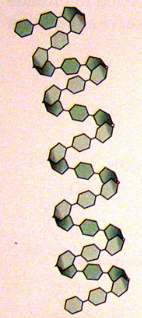
how does the structure of amylose link to its function
helix → compact
polysaccharide= large molecule→ cannot leave cell
large molecule= insoluble→ doesn’t affect water potential
structure of amylopectin
branched
1,4 glycosidic bonds
1,6 glycosidic bonds
coils into helix- held together by hydrogen bonds
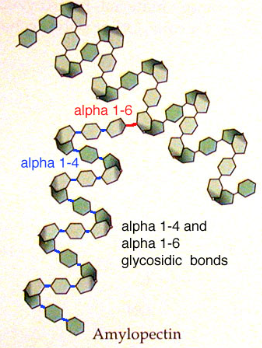
how does the structure of amylopectin relate to its function
helix → compact
polysaccharide= large molecule→ cannot leave cell
large molecule= insoluble→ doesn’t affect water potential
branches → easily hydrolysed by enzymes to release glucose
structure of glycogen
branched
1,4 glycosidic bonds
1,6 glycosidic bonds
coils into helix- held together by hydrogen bonds
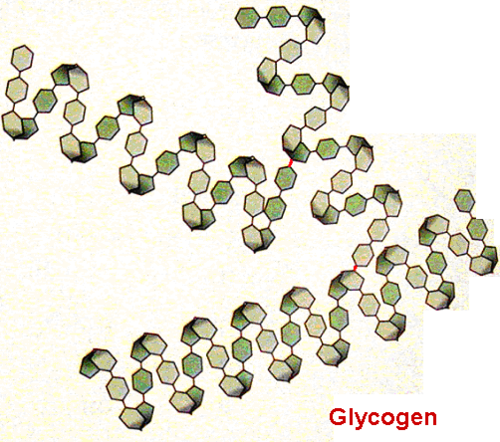
how does the structure of glycogen link to its function
helix → compact
polysaccharide= large molecule→ cannot leave cell
large molecule= insoluble→ doesn’t affect water potential
more branches:
more compact
more ends=easier to remove monomer units (needed as humans have higher metabolic demand)
structure of cellulose
β-glucose molecules bonded together by 1,4 glycosidic bonds
each alternate β-glucose is flipped 180° so it can bond to the adjacent molecule
makes long chains that are unbranched and straight
run parallel with hydrogen bonds (due to many OH groups) between the chains
how is the cell wall formed from cellulose
60-70 cellulose molecules become crosslinked by hydrogen bonds- form microfibrils
around 400 microfibrils are bonded together by H-bonds to form macrofibrils:
have high tensile strength-reduces bursting- embedded in polysaccharide glue (pectin) to form cell walls
macrofibrils run in all directions to increase strength
features of the cell wall
very strong- thousands of chains linked together
fully permeable- allow movement of water and substances to and from membrane due to space between fibrous chains
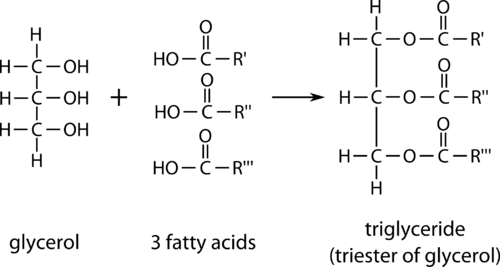
triglycerides
made up of C, H, O
non polar- insoluble in water, so doesn’t affect water potential
soluble in alchohols
structure of triglycerides
3 fatty acid chains ester bonded to a glycerol molecule
formed in condensation reactions
types of triglycerides
saturated- no C=C double bonds
fully saturated with hydrogen
stack neatly into layer so are solid
monounsaturated- 1 C=C double bond
liquid-chains pushed further apart by C=C double bond
form oils
polyunsaturated- many C=C double bonds
liquid- forms oils
roles of lipids
Cell membranes- cell membrane formed of phospholipid bilayer
energy source:
triglycerides broken down in respiration to release energy and make ATP
ester bonds hydrolysed, then glycerol and broken down into carbon dioxide and water
makes more water than respiration of a sugar
waterproofing:
waxes are a type of lipid
fatty acids and alcohols larger than glycerol
insulation:
adipose tissue is a storage location for lipids, slow conductors of heat
lipids in nerve cells act as electrical insulators
buoyancy:
fat is less dense than water, so it floats
protection:
fat around organs absorbs shock
bacteria have peptidoglycan around cells
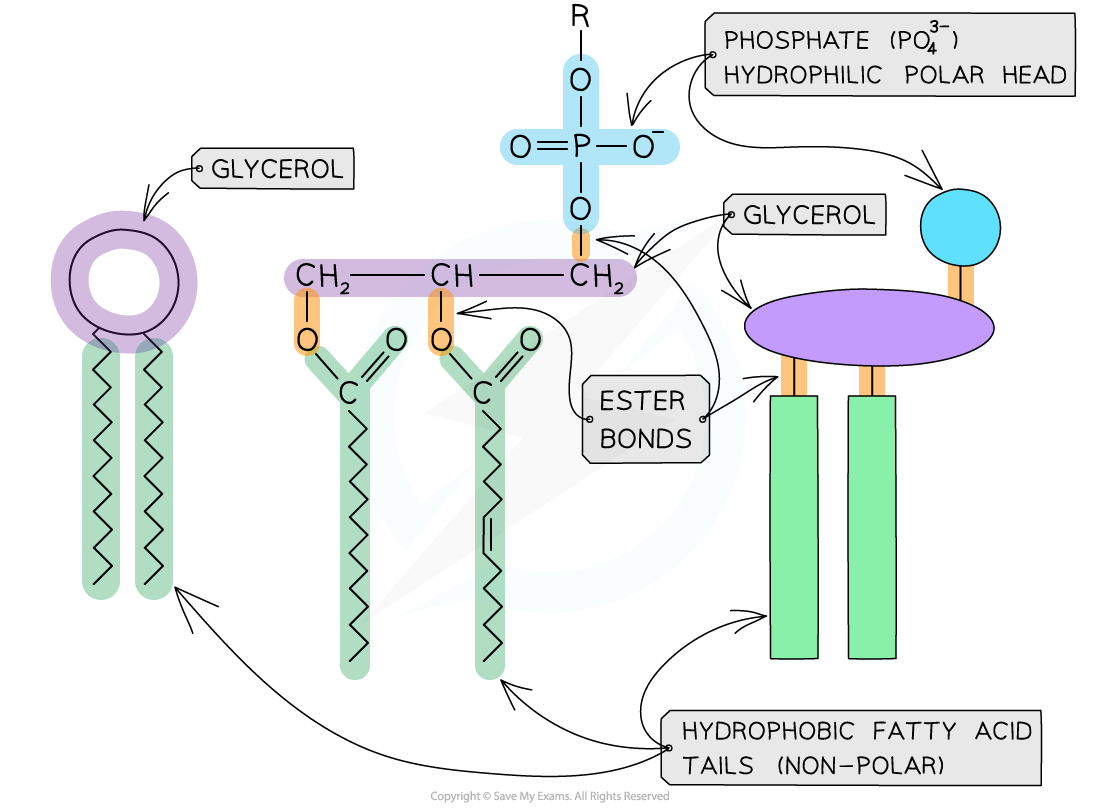
phospholipids
polar molecules
amphipathic- has both hydrophobic and hydrophilic regions
structure of phospholipids
have 2 fatty acids and a phosphate group
phosphate head is a hydrophilic head:
polar-attracted to water
not attracted to fat-orientates towards aqueous solutions
fatty acids are hydrophobic tail:
non-polar→orients away from water
mixes with fat- orientates away from aqueous solution
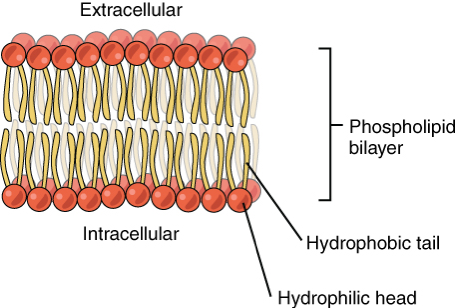
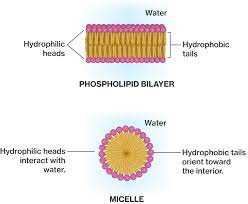
phospholipid bilayer
plasma membrane separates 2 aqueous environments
individual phospholipids can move within their layer, but won’t expose hydrophobic tails to water→ provides stability
selectively permeable → only small non-polar molecules move through
phospholipids in water
arranged in layer on water with heads in water and tails sticking out
OR
form micelles-rings/circles
Glycolipids
the structure of phospholipids allows them to form glycolipids by combining with carbohydrates within cell surface membrane
Important for cell recognition
cholesterol
completely hydrophobic
made of 4 carbon rings
made in liver
function of cholesterol
form steroid hormones e.g. testosterone, oestrogen
pass directly through lipid bilayer → go to nucleus
sits between phospholipid hydrocarbon chain
strengthens cell membrane and regulates fluidity
lipids in plants
produce a derivative of cholesterol found in membranes-stigmasterol
some plant steroids can be converted to animal hormones
roles of proteins
enzymes
hormones and receptors
growth and repair
structural
antibodies
amino acids structure
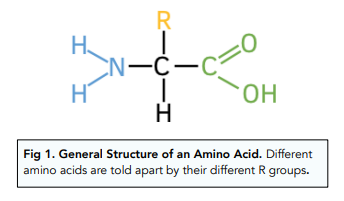
where do amino acids come from
20 naturally occurring amino acids
plants make amino acids using nitrates and products of photosynthesis
animals can make some amino acids but need to ingest others
ingested→ essential amino acids
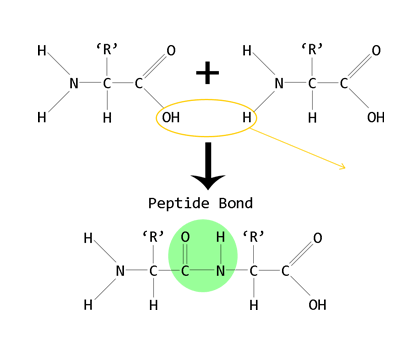
joining amino acids
condensation reaction
levels of protein structure
primary
secondary
tertiary
quaternary
primary structure of proteins
the sequence of amino acids in polypeptides of a protein
less than 50 amino acids= peptides, more than 50= polypeptides/proteins
coded from DNA
consists of peptide bonds between amino acids
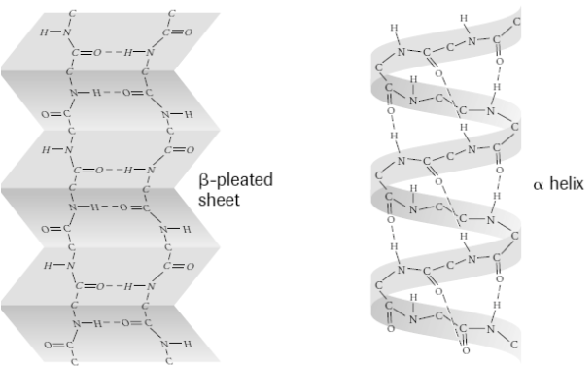
secondary structure of proteins
the way in which proteins are coiled
hydrogen bonds present due to δ+NH group and δ- -C=O group
tertiary structure of proteins
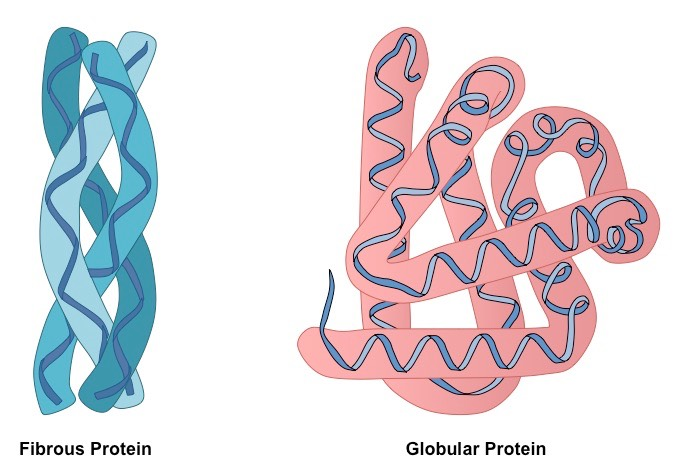
describes 3D shape of proteins- can be:
fibrous
globular
formed due to bonds between R groups of amino acids- bend polypeptide chains
determines function of protein
ionic or disulphide bonds present
Quaternary structure of proteins
proteins may consist of two or more polypeptides
e.g. haemoglobin, antibodies
hydrophobic interactions of proteins
non-polar R groups cluster together in water
weak associations = hydrophobic interactions
when polypeptide chain folds, hydrophobic R-groups tend to be close to each other in interior of folded chain, whereas hydrophilic R-groups tend to be on outside, attracted to water
denaturing proteins
denaturing→ bonds maintaining protein are broken
protein stops functioning properly
fibrous- lose structural strength
globular- insoluble and inactive
happens if temp is above optimum or if pH is not optimum
properties of fibrous proteins
formed of regular and repetitive amino acid sequences- form parallel polypeptide chains held together by crosslinks
long, rope-like fibres
high tensile strength
flexible
insoluble in water (non polar hydrophobic R groups face outwards)
functions of fibrous proteins
structure e.g. collagen
protection e.g. keratin
elasticity e.g. elastin
contraction/ mechanical movement e.g. actin and myosin
properties of globular proteins
spherical shape- tightly folded polypeptide chains
specific tertiary structure
chains folded so hydrophilic R group faces outwards→ soluble
temperature and pH sensitive
functions of globular proteins
transport proteins e.g. carrier proteins
transport substances e.g. haemoglobin
enzymes e.g. pepsin
hormones e.g. insulin
antibodies- destroy pathogens through agglutination
prosthetic groups and examples
a non protein component that forms a permanent part of the functioning protein molecule
e.g. iron in Haem, Zn ion in carbonic anhydrase
conjugate protein and examples
a protein containing a prosthetic group
e.g. haemoglobin carbonic anhydrase
haemoglobin
oxygen carrying pigment found in erythrocytes
4 polypeptide chains in quaternary structure-globins- each has prosthetic haem group:
α-globin
β-globin
four globin subunits held together by disulphide bonds
prosthetic haem group contains Fe2+ ion- can reversibly combine with oxygen to form oxyhaemoglobin
each haemoglobin can carry 4 oxygen molecules, so 8 oxygen atoms
insulin
2 polypeptides
both chains fold into tertiary structure- joined by disulphide links
hydrophilic R groups face on outside- soluble
increase glucose uptake into cells
pepsin
digests protein in stomach
single polypeptide
few basic R groups, many acidic R groups- more stable in acidic environment- few basic groups to accept H+ ions- little effect on structure.
keratin
rich in cysteine- many disulphide bridges and hydrogen bonds- strong molecule
found in nails, hair, skin, claws etc
provides mechanical strength
impermeable and waterproof barrier- prevents infection and waterborne pollutants
elastin
found where stretch is important e.g. skin, alveoli, blood vessels, lungs, bladder
stretch and recoil due to crosslinks and coils
collagen
provides mechanical strength:
arterial walls
tendons connect muscles to bones
cartilage and connective tissue
bones made of collagen reinforced with calcium phosphate
3 chains- H bonds between chains
forms crosslinks with other collagen molecules to form collagen fibrils
lots of fibrils=collagen fibre
cations (5)
Ca2+
Na+
K+
H+
NH4+
Anions (5)
NO3-
HCO3-
Cl-
PO43-
OH-
Calcium ions
increases rigidity of bones, teeth and cartilage
component of crustacean exoskeleton
enzyme activator
stimulates muscle contraction and regulates nerve transmission
regulates cell membrane permeability
important for cell wall development in plants