General Chemistry 131 Final Exam
1/178
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
179 Terms
Matter
Anything that occupies space, tangible, anything with mass
Mass
The amount of matter in an object
Physical Quantities
Mass=kilograms (kg)
Length=meter (m)
Time=seconds (s)
Amount=moles (mol)
Temperature=kelvin (K)
Kilo
k
1 kg=10³ grams
Centi
c
1 cg=10⁻² grams
Milli
m
1 mg= 10⁻³ grams
Micro
(weird u shape)
1 microgram= 10⁻⁶ grams
Nano
n
1 ng= 10⁻⁹ grams
Precision
How close together measurements are
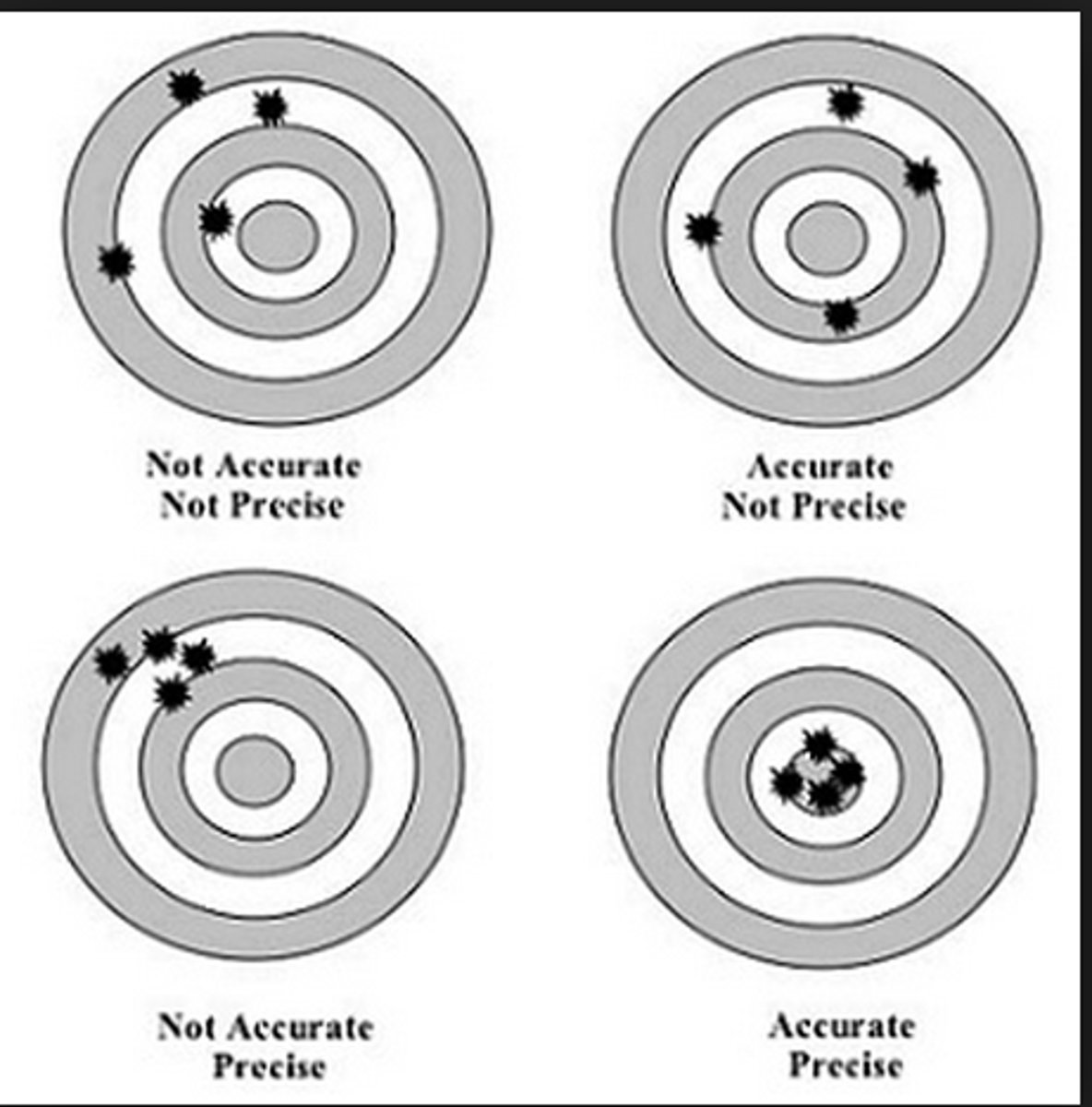
Accuracy
How close a set of measurements are to the true value
Significant Figures (SigFigs)
The number of meaningful certain digits and one uncertain digir
Rules for Sig Figs
1) Zeros in the middle of the number, like any other digit, are significant
2) Zeros at the beginning of a number are not significant, they simply tell where the decimal point is.
3) Zeros at the end after the decimal are always significant; zeros wouldn't show unless they were significant
4) Zeros at the end may or may not be significant, they might be apart of the measurement or locate the decimal
5) Certain numbers are exact
To report correct sig figs on digital read-
All digits are significant and should be reported, ALWAYS report from the bottom of the meniscus
To report correct sig figs on non-digital reads-
Report one digit past the lines on the measuring device, report at the bottom of the meniscus
Rules for Rounding
-If the first digit you remove is less than 5, round by dropping it and all following digits.
-If the first digit you remove is more than 5, round up by adding 1 to the last kept digit.
Keeping Sig Figs in Math Operations
Multiplication and Division
-The answer CANNOT have more sig figs than any of the original numbers
-The answer has to have the same number of sig figs as the number used with the least number of sig figs.
Addition and Subtraction
-After lining up the decimal points, the answer can't have more sig figs to the right than any of the original numbers
Performing Multiple Steps
-Keep track of the number of sig figs in each step
-Round at the end
Equation for Density
Mass (g)/Volume (mL or cm³)
Celsius to Farenheit
(9°F/5°C) x °C +32°F
Fahrenheit to Celsius
(5°C/9°F) x (°F-32°F)
Temperature in Kelvin
°C+273.15°
Temperature from Kelvin to Celsius
K-273.15°
Energy
The ability to do work or the ability to supply heat
Total Energy (E)
Kinetic energy+Potential energy
E(k)=kinetic, energy of motion
E(p)=potential, potential to supply energy (stored energy)
Kinetic Energy E(k)=
(1/2)mv²
m=mass (kg)
v=volume (d/t), (m/s)
(1/2) kg x (m/s)²
Energy Conversions
1 cal=4.184 J
1 Cal=1000 cal
1 Cal= 1 kc
1 J=1000 kJ
The periodic table is organized by...?
Number of protons, ATOMIC NUMBER
Periods on the Periodic Table
Rows, properties of elements change a lot moving across the rows
Groups on the Periodic Table
Also called families, columns, properties are similar within a column (top to bottom)
Chemical VS. Physical Properties
Chemical:
-Describes a substance reacting (or not) to form other substances
-Iron rusting, something burning, tarnishing metal
Physical:
-Does not involve a change in identity of a substance
-Color, Ice melting
-Boiling and melting point, paper tearing, malleability, size, conductivity
Extensive VS. Intensive Properties
Extensive:
-Depends on SIZE, however, density is NOT extensive
Intensive:
-Doesn't depend on SIZE
-Color, metal rusting, burning wood, DENSITY, wood floating on water
Properties of Group 1
Alkali Metals
-Soft, silvery metals
-NEVER found in elemental forms in nature
-Make salts
-Elemental forms react rapidly and violently w water
-More reactive toward bottom of column
Properties of Group 2
Alkaline Earth Metals
-Silvery metals
-NEVER found in elemental forms in nature
-Make salts
-Less reactive than Alkali metals
-Make basic solutions in water
-More reactive towards bottom of column
Properties of Group 7
Halogens
-NEVER found in elemental forms in nature
-Make salts
-Colorful, corrosive non-metals, except for astatine (semimetal)
Properties of Group 8
Noble Gases
-Typically found in nature
-Very inactive
-Colorless gases
Element
A fundamental substance that can't be broken down into anything simpler
Main Groups
Two larger groups on the left and the six larger groups on the right (Groups 1,2,13,14,15,16,17,18)
Transitional Metal Groups
10 smaller middle groups (3-12)
Inner-transition Metal Groups
14 groups below the main periodic table
Characteristics of Metals
Solid at room temp (except Mercury), most have a silvery shine, malleable NOT brittle, good conductors of heat and electrivity
Characteristics of non-metals
None are silvery, some are colorful, brittle, do not conduct heat or electricity
Characteristics of Semimetals
Most are silvery in appearance, solid at room temperature, brittle, poor conductors of heat and electricity
Chemical Compounds
Atoms joining together in different ways to create a vast number of substances
Chemical Formula
lists the symbols of the constituent elements and uses subscripts to indicate the number of atoms in each
Chemical Equation
in which the reactant substances undergoing change are written on the left, products on the right, and arrows show the direction of the reaction
Law of Mass Conservation
Mass is neither created nor destroyed in a chemical reaction
Law of Definite Proportions
Different samples of pure chemical compound always contain the same proportion of the elements by mass
Law of multiple proportions
Elements can combine in different ways to form different chemical compounds, whose mass ratios are simple whole numbers that are multiples of each other
Elements are categorized by...?
the mass of its atoms
Alpha particles
type of emission given off by a number of naturally occurring elements, 7000 times more massive than an electron. Charge=2e⁻
Nucleus
Mass concentrated in a central core of the cell
Protons
Positive charge (# of protons and electrons in an atom are equal)
Neutrons
No charge
Atomic Number
number of protons in an atoms nuclei, # of electrons
Mass number
the sum of the protons and the neutrons in the nuclei
Unified Mass Unit
u
Atomic mass unit, (amu)
Atomic Mass
Mass of a specific atom
Atomic Weight
Weighted average of the atomic masses of the elements naturally occurring isoptopes
Mole
One mole of any element is the amount whose mass in grams called MOLAR MASS is equal to atomic weight
Equation for Average Atomic Mass
∑(Mass of isotope(%/100%))
Principal Quantum Number
n
Ranges from one to infinity in integer values. As n increases, the radius of the orbital increases
n↑, size↑
Angular momentum
L
Ranges from 0 to n-1
Integer values and whole numbers
Defines the shape
each value has a letter abbreviation
Equation for Change in Energy
∆E=E(final)-E(initial)
Positive change in energy means...?
Energy is required/put in for the reaction to take place, occurs when n is increasing
Negative Change ion energy means...?
Energy is released, let out, when n is decreasing
Ground state
e⁻ in the lowest energy orbital, MOST STABLE STATE OF THE ATOM
Excited State
e⁻ not in the lowest orbital, not stable
Wavelength
λ
The distance or length of one repeating unit of a wave (meters)
Frequency
v
# of repeating units that pass a point in a specified amount of time
Equations to find Light
v=c/λ
E=hv=hc/λ
Orbitals
indicate where the e⁻ is likely to be
-each orbital corresponds to a particular energy
-waves of electrons
Angular Momentum (L)= 0
s orbital
sphere shape
Angular Momentum (L)=1
p orbital
dumbell shape
Angular Momentum (L)=2
d orbital
double dumbbell shape
Angular Momentum (L)=3
f orbital
undefined shape
Magnetic Quantum Number M(l)
ranges from -l to l passing through 0
each value corresponds to an orbital w a different orientation in space
If n=1...?
l=0
M(l)=0
Orbital=1s
Number of orbitals in shell=1
If n=2...?
l=0, 1
M(l)= -1,0,1
Orbital=2s or 2p
If n=3...?
l=0,1,2
M(l)=-2,-1,0,1,2
Orbital=3s,3p,3d
If n=4...?
l=0,1,2,3
M(l)=-3,-2,-1,0,1,2,3
Orbitals=4s,4p,4d,4f
Spin Quantum Number
M(s)
+1/2 or -1/2
Effective Nuclear Charge
Zeff
Charge from the nucleus that the e⁻ actually "feels"
=Zact-S
Zact=# of protons
S=Shielding (repulsions from other e⁻)
Zeff↑,size↓
Afbau Principle
-Lower energy orbitals fill first, before higher energy orbitals
-an orbital cal only hold 2 electrons and they must have opposites spins
-If two or more degenerate orbitals are available, one e⁻ goes to each until each are half full
S block
Li and Be down, n being filled matches period #
P block
B→He/Ne down
n being filled matches period #
D block
Sc→Zn and down
n=period #-1
F block
La→Yb and down
n= period #-2
Relationships between energy, period, and size
Zeff↑ moving left to right across the table
F: High for EN, Eea, Ei, Zeff
Low for size/radius,volume
Top to bottom: n↑, ↑radius, size
Left to right: ↑Zeff, ↓Radius, size
Ionization Energy
E(i)
Energy required to remove an electron from an atom
Requires energy
Left to right: Zeff↑, E↑
Electron Affinity
E(ea)
Energy involved in adding an electron to an atom
Larger Tea is more negative, more favorable to gain an electron
Negative ion
anion
gains e⁻
Positive ion
cation
loses e⁻
The Octet Rule
Main groups tend to undergo reactions that leave them with a total of 8 or 0 electrons in their valance s and p orbitals
The Octet Rule regarding H and He
These will undergo reactions that leave them with 2 or 0 valence s orbitals
When losing electrons...
p block- loses p e⁻s then s e⁻s
s block- loses s e⁻s
d block- lose s e⁻s then de⁻s
f block- loses s e⁻s then f e⁻s
Covalent Bonds
No metals or NH₄⁺
Involve sharing of electrons
Between two nonmetals, two semimetals, or one of each
Polar Covalent Bond
Two different elements (NOT C-H bonds)
Non-Polar Covalent Bonds
Two of the same elements bonded together AND C-H bonds
Elements that form Covalent bonds
-Nonmetals and semimetals
-Metals usually don't form covalent bonds
-Compounds held together just by covalent bonds with no charge are called molecules
Molecular Elements
-Elements found as molecules with covalent bonds between atoms
-Found in upper right hand corner
-H₂,N₂,O₂,F₂,Cl₂Br₂,I₂
Ionic Bonds
Occurs between metal or NH₄⁺ and something
Held together by oppositely charged ions
Have a lattice structure