Hon. Chem Ch. 3 Atoms: The Building Blocks of Matter
0.0(0)
Card Sorting
1/53
Earn XP
Description and Tags
Last updated 12:01 PM on 10/27/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
1
New cards
Atom
smallest particles of an element that maintains the chemical properties of that element. Neutral when protons= electrons
2
New cards
Law of Conservation of Mass
mass is neither created nor destroyed during ordinary chemical reactions or changes; can only change form
3
New cards
Scientist for Law of Conservation of Mass
Lavoisier
4
New cards
Example of Law of Conservations of Mass
wood burning in a fire; wood changes to ashes, yet still has same mass
5
New cards
Law of Definite Proportions
a single chemical compound contains the same elements in exactly the same proportions by mass, regardless of the size of the sample or the source of the compound
6
New cards
Scientist for Law of Definite Proportions
Proust
7
New cards
Examples of Law of Definite Proportions
sodium chloride(table salt) always consists of 39.34% by mass of the element sodium (Na), and 60.66% by the mass of the element chlorine (Cl);
Water molecule: 2 hydrogen, 1 oxygen atoms = 11% hydrogen and 89% oxygen
Water molecule: 2 hydrogen, 1 oxygen atoms = 11% hydrogen and 89% oxygen
8
New cards
Law of Multiple Proportions
if 2 or more compounds are composed of the same 2 elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole numbers
9
New cards
Scientist for Law of Multiple Proportions
Dalton
10
New cards
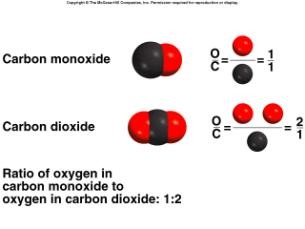
Examples of Law of Multiple Proportions
carbon and oxygen (CO2)
carbon dioxide and carbon monoxide (CO)
Consider samples of each of these compounds, each containing 1.00 g of carbon. In carbon dioxide, 2.66 g of oxygen combine with 1.00 g of carbon. In carbon
monoxide, 1.33 g of oxygen combine with 1.00 g of carbon. The ratio of the masses of oxygen in these two compounds is 2.66 to 1.33, or 2 to 1.
carbon dioxide and carbon monoxide (CO)
Consider samples of each of these compounds, each containing 1.00 g of carbon. In carbon dioxide, 2.66 g of oxygen combine with 1.00 g of carbon. In carbon
monoxide, 1.33 g of oxygen combine with 1.00 g of carbon. The ratio of the masses of oxygen in these two compounds is 2.66 to 1.33, or 2 to 1.
11
New cards
Dalton's Atomic Theory
1) all matter is made of small particles called atoms
2) atoms of an element are identical in size, mass, and other properties*
3) atoms can't be subdivided* , created or destroyed
4) atoms of different elements combine in simple or whole number ratios to form compounds
5) in chemical reactions, atoms are combined, separated, or rearranged
2) atoms of an element are identical in size, mass, and other properties*
3) atoms can't be subdivided* , created or destroyed
4) atoms of different elements combine in simple or whole number ratios to form compounds
5) in chemical reactions, atoms are combined, separated, or rearranged
12
New cards
Dalton's Atomic Theory are incorrect now
2) atoms of an element are identical in size, mass, and other properties
3) atoms can't be subdivided
3) atoms can't be subdivided
13
New cards
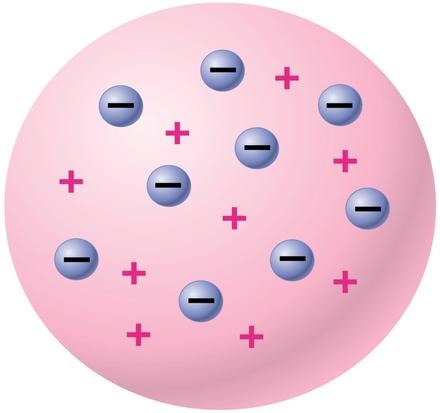
Thomson's Cathode Ray Tube Experiment
Observations:
Rays were deflected away from negatively charged particles
Conclusions:
Particles that composed the cathode ray tube are negatively charged; discovered the electron
Rays were deflected away from negatively charged particles
Conclusions:
Particles that composed the cathode ray tube are negatively charged; discovered the electron
14
New cards
Inference based on Thomson's Model
Atoms are electrically neutral, so atoms must contain a positive charge to balance the negative electrons
15
New cards
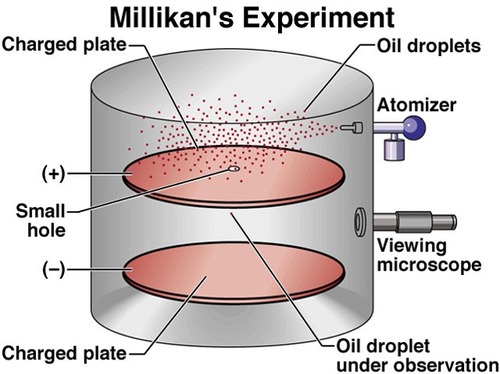
Milikan's Oil Drop Experiment
Observations:
Measured the charge of an electron
Conclusion:
Scientists used this information and the charge to mass ratio to determine the mass of an electron
(mass of electron is 9.10*10^-28 g)
Measured the charge of an electron
Conclusion:
Scientists used this information and the charge to mass ratio to determine the mass of an electron
(mass of electron is 9.10*10^-28 g)
16
New cards
Inference Based on Milikan's Experiment
Since electrons have almost no mass, atoms must contain other particles that account for the mass of an atom
17
New cards
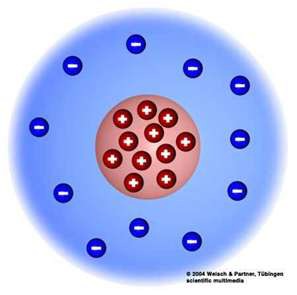
Rutherford's Gold Foil Experiment
Observations:
1 in 8000 particles were deflected back
Conclusions:
The alpha particles hit something small and dense; discovered nucleus
1 in 8000 particles were deflected back
Conclusions:
The alpha particles hit something small and dense; discovered nucleus
18
New cards
Nuclear Forces
the interaction that binds protons and neutrons, protons and protons, and neutrons and neutrons together in a nucleus
19
New cards
Proton Charge
+1 (positive)
20
New cards
Proton Mass
1u
21
New cards
Proton symbol
p+
22
New cards
Proton Location
nucleus
23
New cards
Neutron Charge
0 (neutral)
24
New cards
Neutron Mass
1u
25
New cards
Neutron symbol
n
26
New cards
Neutron location
nucleus
27
New cards
Electron charge
-1 (negative)
28
New cards
Electron mass
0
29
New cards
Electron symbol
e^- (e-)
30
New cards
Isotopes
atoms of the same element (same number of protons and electrons) that have different masses (different number of neutrons)
31
New cards
Nuclide
a general term for a specific isotope of an element
32
New cards
Isotopes of Hydrogen
protium, deuterium, tritium
33
New cards
Protium
isotope of hydrogen with 1 proton and 1 amu; stable

34
New cards
Deuterium
isotope of hydrogen with one proton and one neutron in the nucleus; stable

35
New cards
Tritium
isotope of hydrogen with 1 proton, 2 neutrons, 3 amu; radioactive

36
New cards
Hyphen Notation
Element name-mass number; ex. carbon-12
37
New cards
Nuclear Symbol
superscript (mass number), subscript (atomic number) and element symbol
38
New cards
Atomic Number
number of protons and number of electrons
39
New cards
Number of Neutrons
mass number - atomic number
40
New cards
Radioactive Decay
the spontaneous disintegration of a nucleus into a light nuclei, accompanied by particles, electromagnetic, radioactive, or both
41
New cards
Alpha Decay
4/2 He
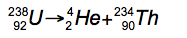
42
New cards
Alpha Particles
radioactive decay; stopped by paper
43
New cards
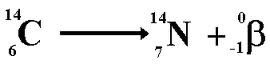
Beta Decay
0/-1 e (B)
44
New cards
Beta Particles
stopped by aluminum foil, radioactive decay
45
New cards
Positron Emission
0/+1 e (B)
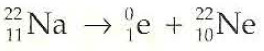
46
New cards
Positron Particles
stopped by aluminum foil, radioactive decay
47
New cards
Gamma Emission
0/0 y
48
New cards
Gamma Rays
stopped by led barrier, radioactive decay, no mass, energy added, most penetrating
49
New cards
Average Atomic Mass
weighted average of the atomic masses of all naturally
to solve, multiply amu by decimal percent and do it for all of them. then add all of them together w/ correct sigfigs
to solve, multiply amu by decimal percent and do it for all of them. then add all of them together w/ correct sigfigs
50
New cards
Mole
the amount of a substance that contains Avogadro's number of particles
51
New cards
Molar Mass
the mass of one mole of a pure substance; units: g/mol
52
New cards
Mole to Atom Conversion (Avogadro's number)
Use 6.022 x 10^23 (Avogadro's number) particles to convert moles to atoms
53
New cards
Mole conversions:
Know how to do:
Moles to mass (g)
Mass (g) to moles
Moles to mass (g)
Mass (g) to moles
54
New cards
Atom Conversions:
Know how to do:
Moles to atoms
Atoms to moles
Mass (g) to moles to atoms
Atoms to moles to mass (g)
Moles to atoms
Atoms to moles
Mass (g) to moles to atoms
Atoms to moles to mass (g)