Subatomic Particles
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Electrons (e-)
Negtivley charged subatomic particle, make up majority volume but mass in negligible
Which subatomic particle mass is negligible?
electron
Valance electrons
Electrons in the outer shell of an atom
Protons (p+)
Positively shared subatomic particles.
Neutrons (n0)
Subatomic partial with no charge
Which subatomic particles make up the Nucleus?
protons and neutrons
Isotopes
Atoms of an element that contain the same numbers of protons but not neutrons.
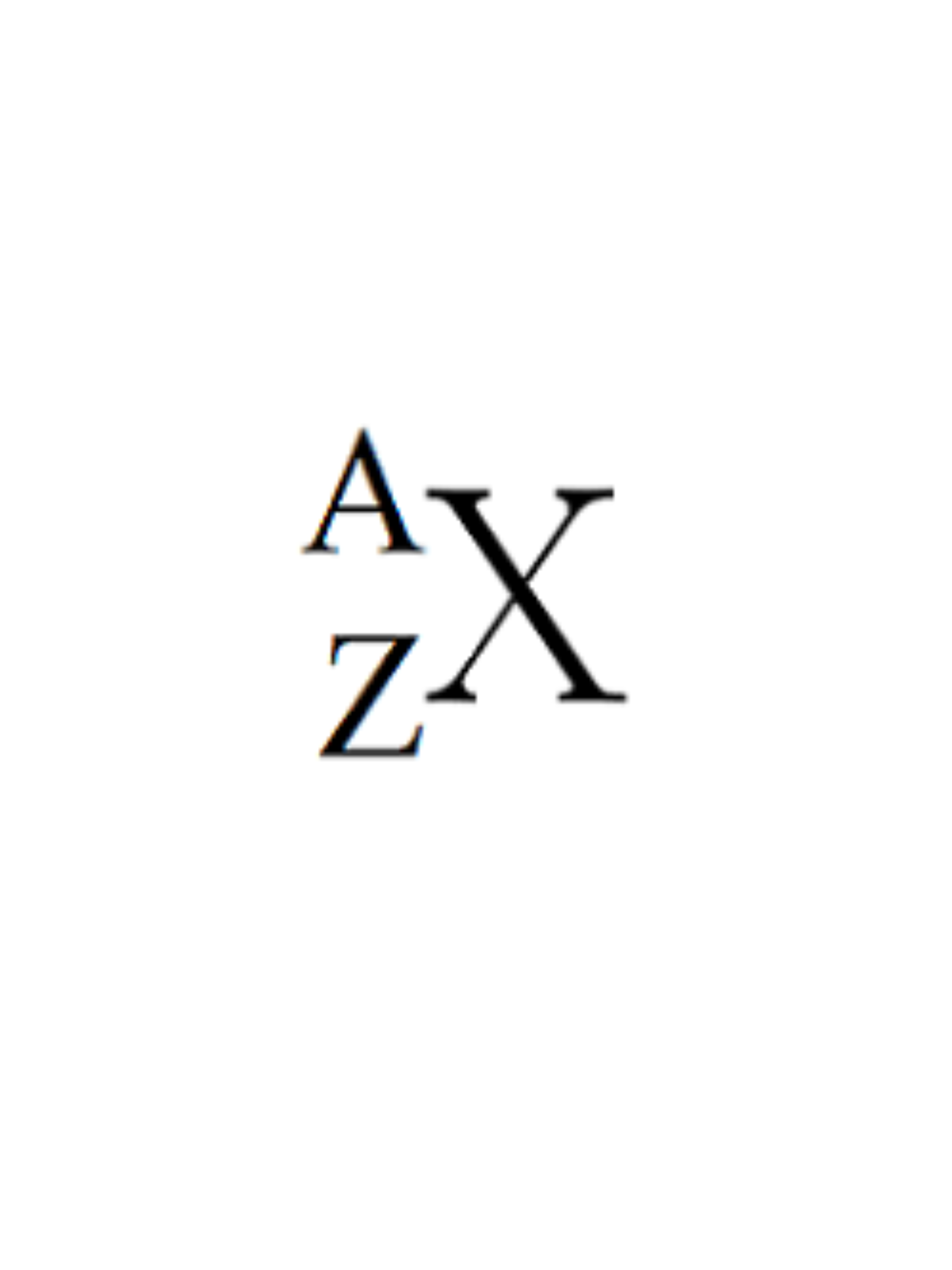
What does X represent?
The symbol for an element
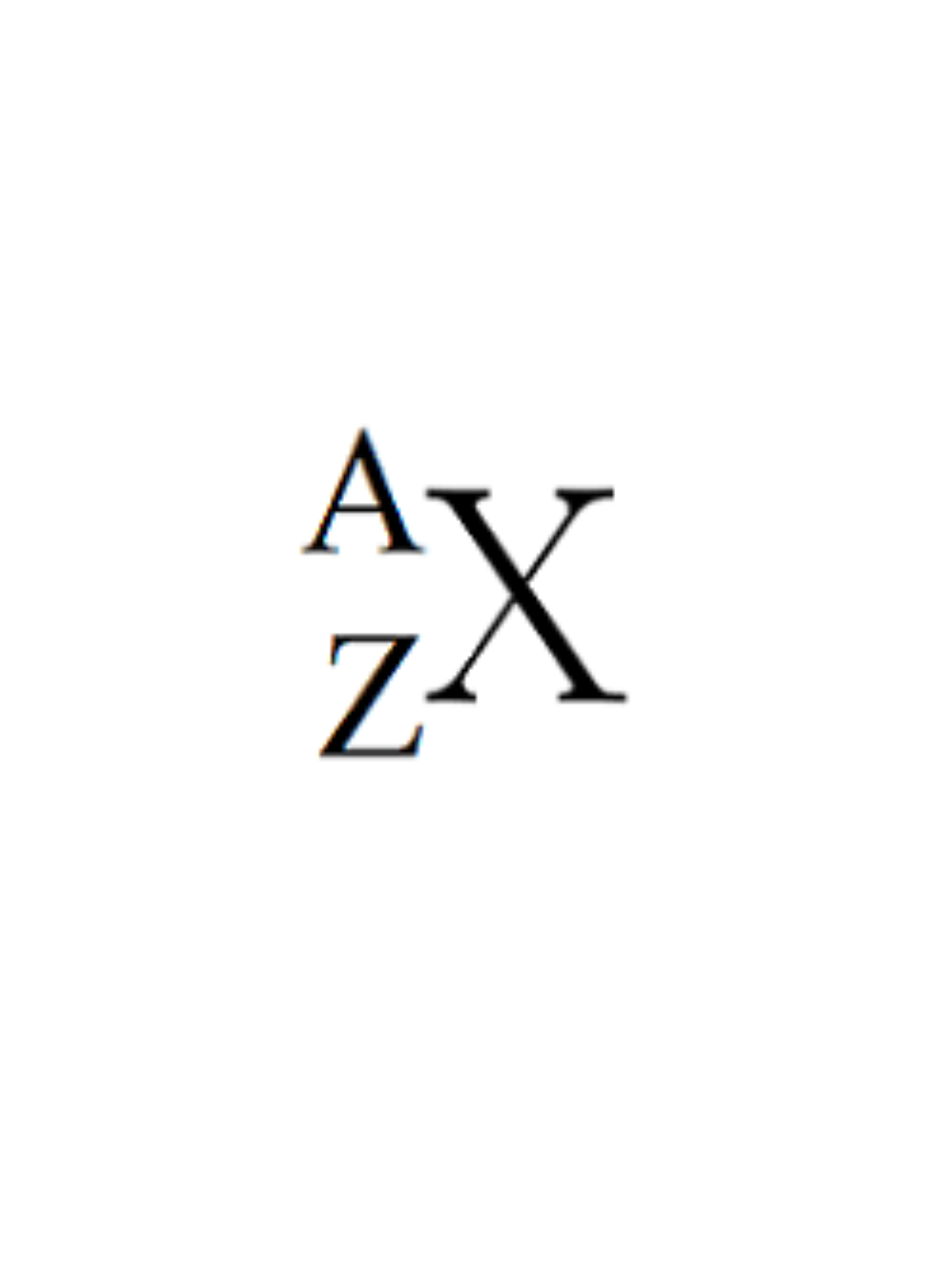
What does A represent?
Atomic mass (# Protons + # Neutrons)
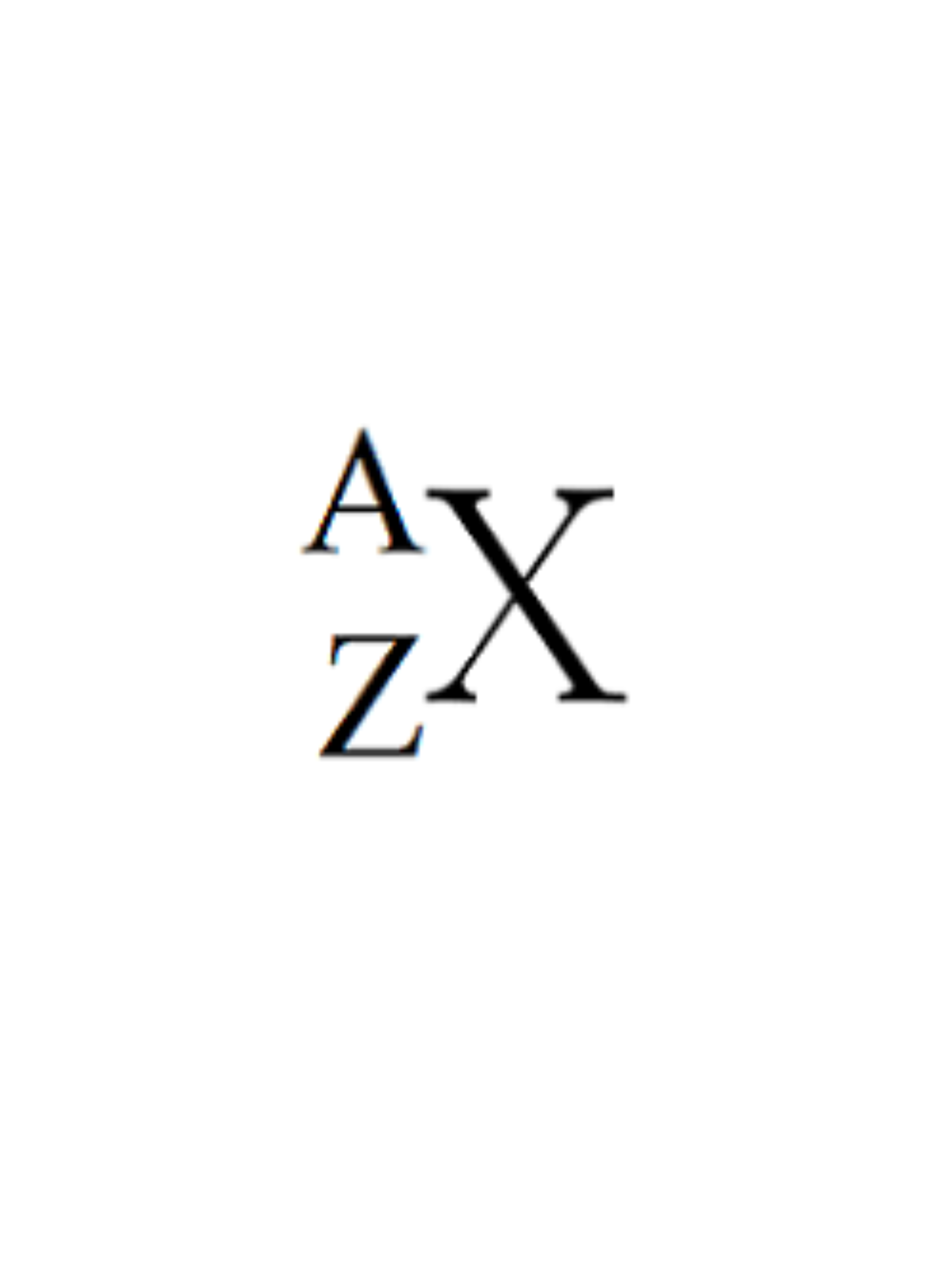
What does Z represent?
Atomic Number (# Protons)
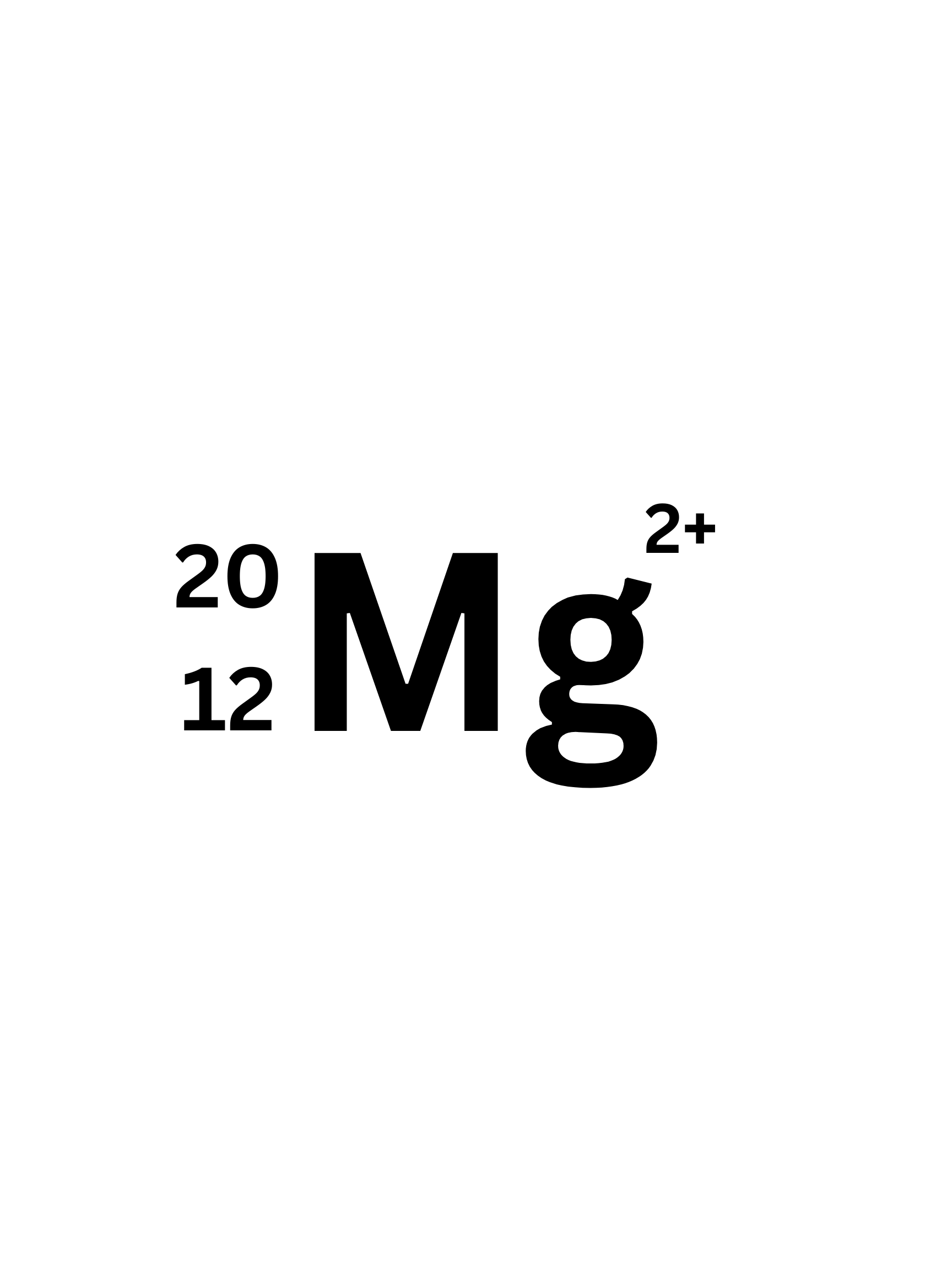
How many Neutrons does this Isotope have?
8 (20-12=8)
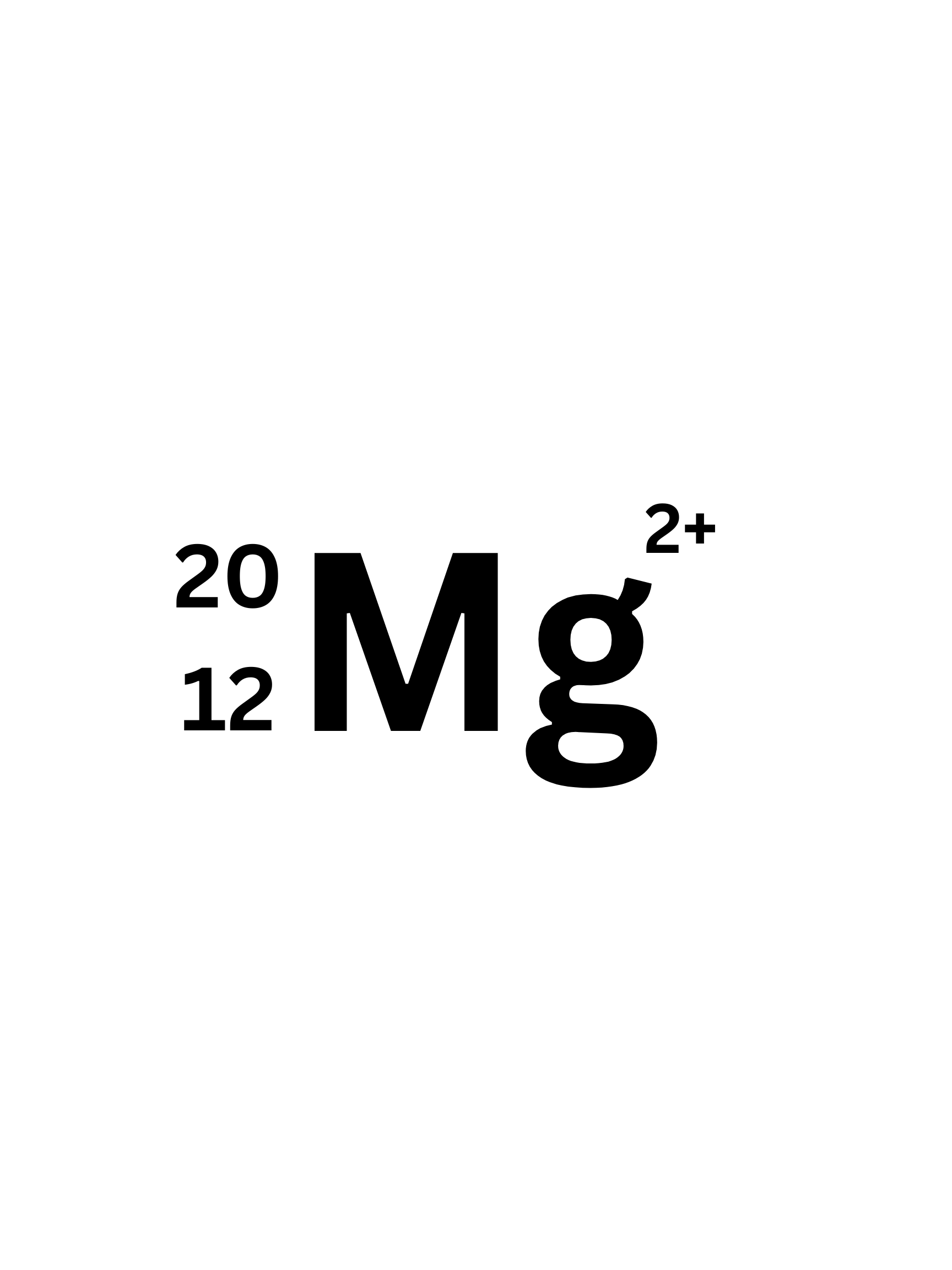
How many protons does this Isotope have?
12
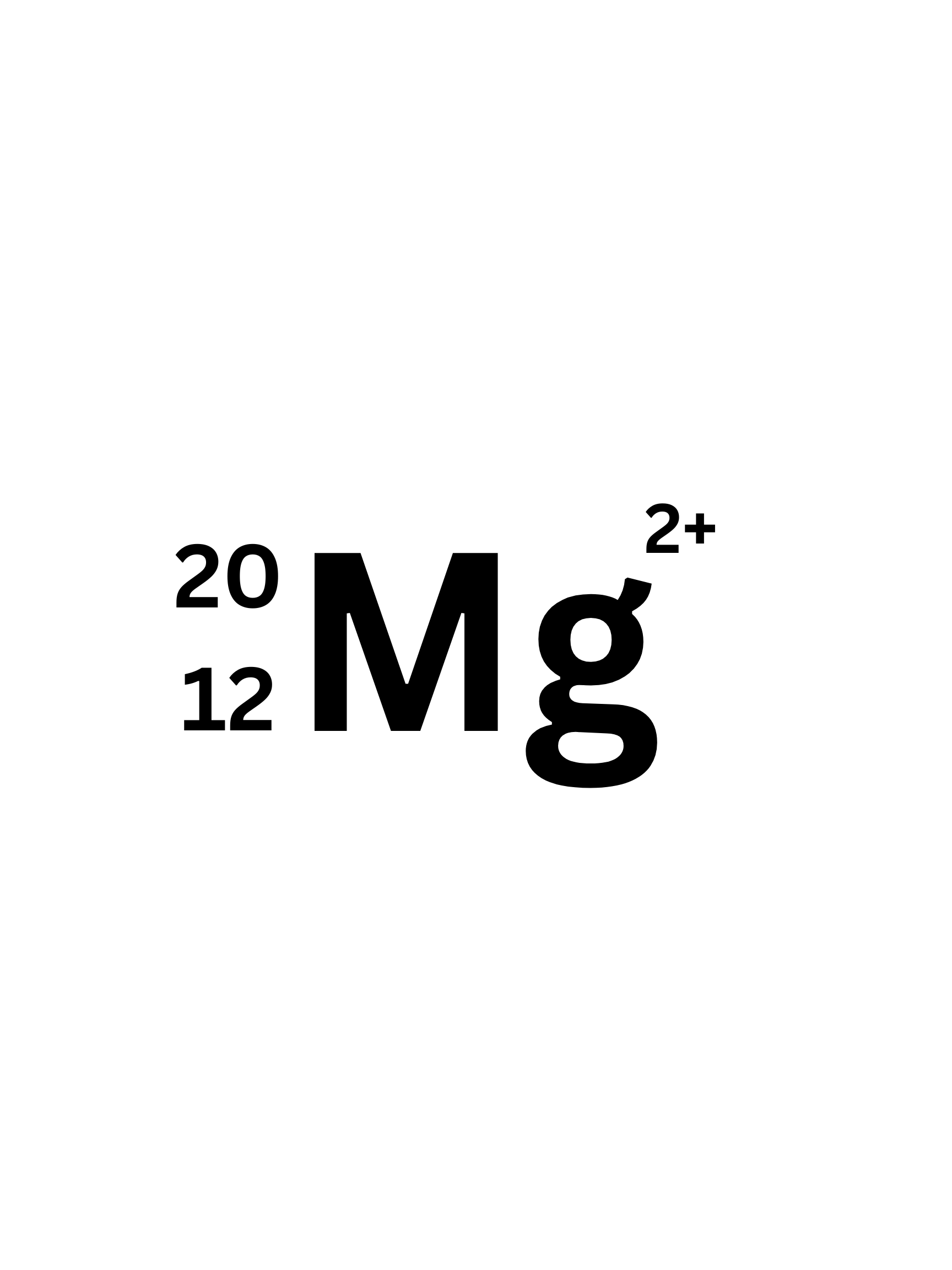
What is the Atomic number of this isotope?
12
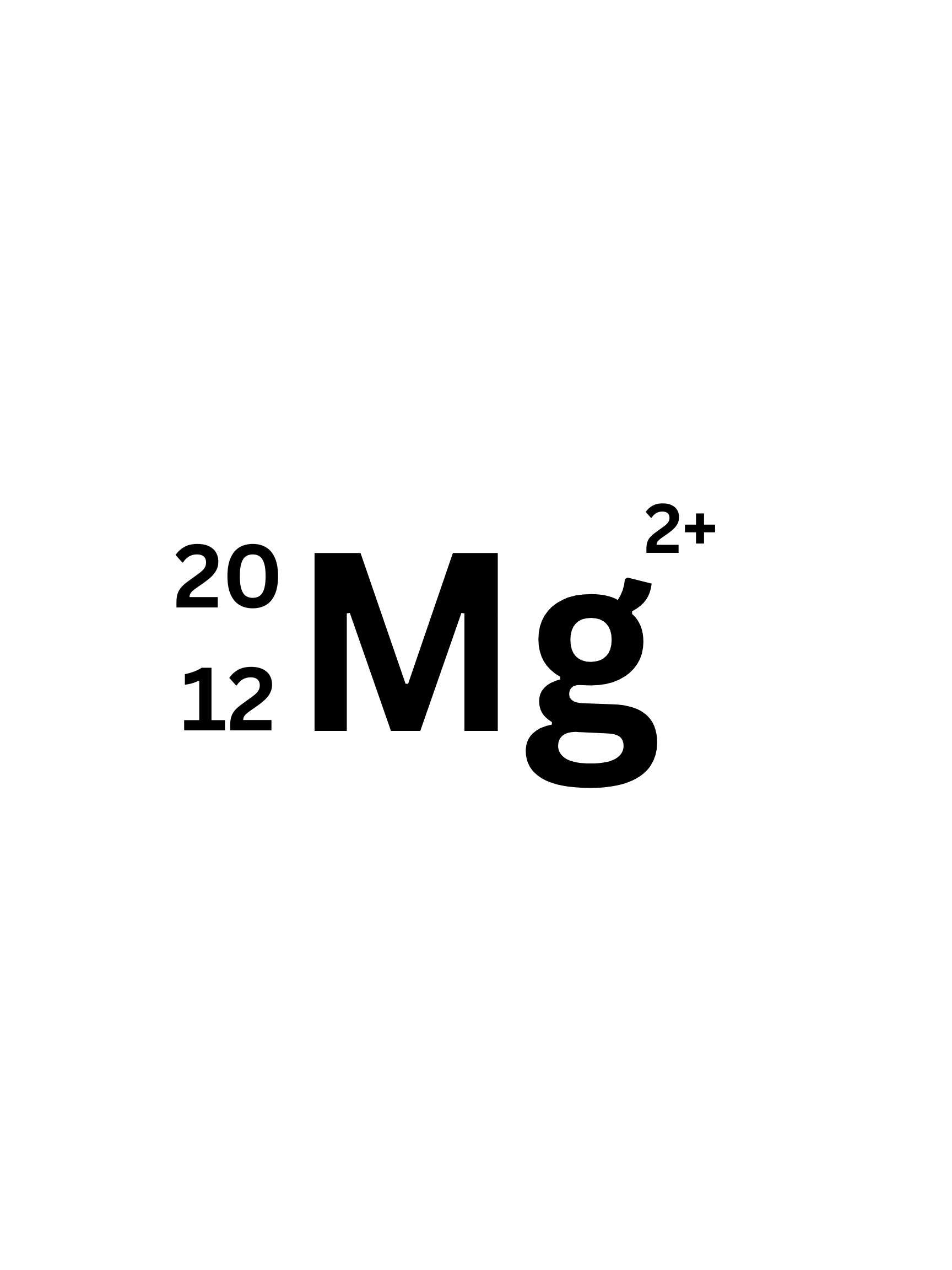
How many electrons does this isotope have.
10 (to have a 2+ charge it lost two electrons)
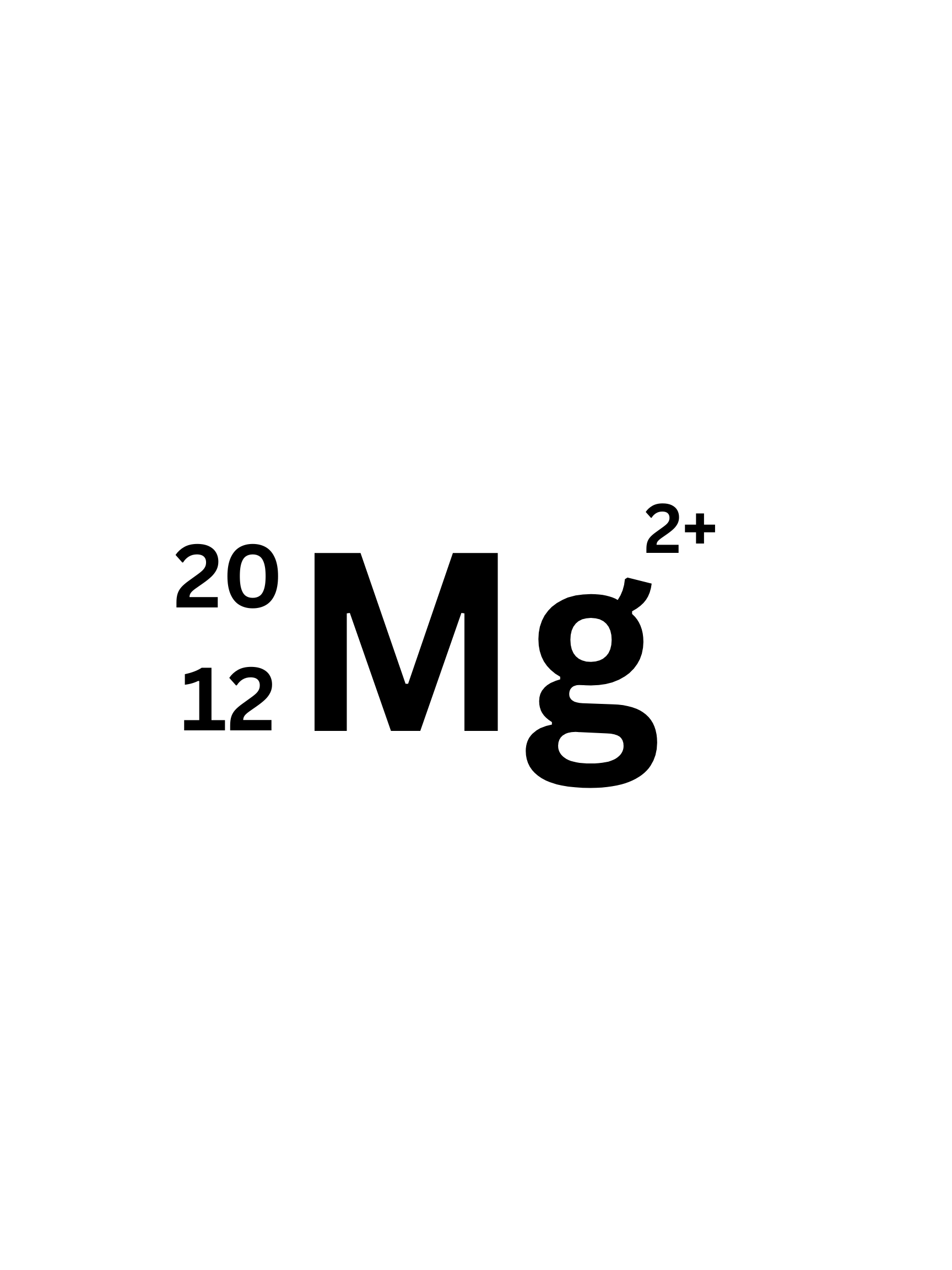
What is the mass number of this isotope?
20
Neutral Atom
# protons = # electrons
Ions
Atoms that either gained or lost electrons giving them a charge
An ion with a positive charge ____ electrons
lost
An ion with a negative charge ____ electrons
gained
Cations
A ion that lost giving it a positive charge
How do I remember that cations are positive?
They make me happy
Anions
An ion that gained electrons giving is a negative charge?
How do I remember that Anions are negative
“an” antagonist, antagonist are evil, evil=negative
Diatomic Molecules
Two atoms covalently bonded
What are the Diatomic Molecules?
H2 N2 F2 O2 I2 Cl2 Br2
What is the acronym for the Diatomic Molecules?
Have No Fear Of Ice Cold Beer
Isometrics
Atoms with the same electron configuration