CHEM111 - molecular geometry
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
sp3 hybridised shape
tetrahedral
sp2 hybridised shape
trigonal planar
sp hybridised shape
linear
isomers
molecules with the same molecular formula but different structure
three types of constitutional isomers
chain, positional, functional group
stereoisomers
same order of attachment of atoms but a different spatial arrangement
chiral
molecules that are non-superimposable on their mirror image
achiral
molecules that are identical to their mirror image
most common form of chiral molecule
tetrahedral carbon bonded to four different groups
why are enantiomers difficult to isolate
they have identical physical and chemical properties
how are enantiomers labelled
right or left handed
R/L handed notation for chiral centres
R or S
R/L notation for metal ligand complexes
delta or lambda
R/L notation for helical molecules
P or M
how can enantiomers be identified
through rotation of the plane of polarised light
how is rotation of plane of polarised light to the left indicated
(-)
how is rotation of plane of polarised light to the right indicated
(+)
rotation of plane of polarised light in racemic mixture
no rotation
racemic mixture
1:1 mixture of two enantiomers
diastereomers
molecules that are non-superimposable non mirror images
notation for distinguishing diastereomers
E and Z
E notation means
two highest priority groups on opposite sides of the double bond
Z notation means
two highest priority groups on the same side of the double bond
how to determine the number of possible stereoisomers, for a compound with n chiral centres
2^n
Fischer projection
a 2D representation of the configuration of chiral molecules
meso isomer
achiral, contains at least two stereocentres and has internal symmetry so it is optically inactive
fractional recrystallisation
mixing cation-enantiomers with a chiral anion to get diastereomeric salts which can be separated by crystallisation
chiral chromatography
natural separation of diastereomers through a column containing starch
kinetic resolution
using an enzyme that prefers the reaction of one diastereomer over another
asymmetric catalysis
reaction so that only one enantiomer is produced
conformation
arrangements of the molecule with the same molecular formula but there’s free rotation around each bond in the molecule
preferred conformers are
lower in energy
why is changing conformation relatively lower energy than changing configuration
changing configuration involves the breaking and reformation of bonds
different conformations of a molecule might have different…
behaviours and properties
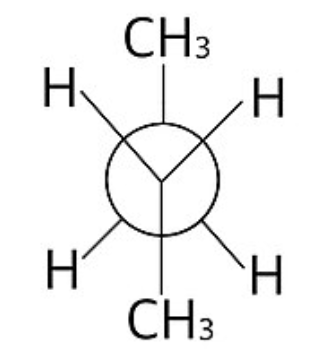
staggered
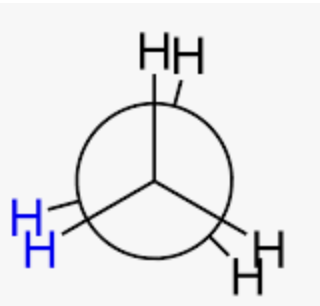
eclipsed
why is staggered the preferred conformer
it minimises steric clash and electron repulsion
how is the staggered form stabilised
interactions between filled and unfilled orbitals
what notation/drawing is used for cycloalkanes like cyclohexane
chair and boat
axial
groups above and below the plane of the ring
equatorial
groups sticking out from equator plane of the ring
what conformation is every C-C bond in cyclohexane
staggered
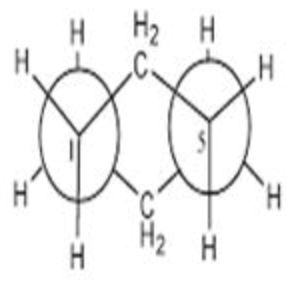
staggered cyclohexane
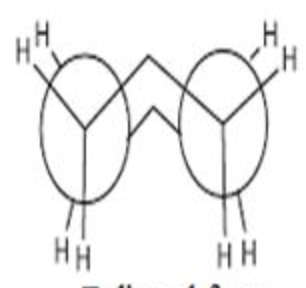
eclipsed cyclohexane
why is free rotation not possible in the ring system
restricted rotation about the C-C bonds
what is the most stable conformation for monosubstituted systems
where the substituent is in the equatorial position because axial would be destabilised by other interactions
what is the steric preference for larger substituent groups
have them further away from each other
what happens to the chair forms in solution
they exist in equilibrium with each other
disubstituted cyclohexanes can result in
diastereomers
both cis conformers and both trans conformers have the same
energy
what conformation is preferred for disubstituted cyclohexanes with two different substituted groups
where the larger group is equatorial