enthalpy changes and calorimetry
1/11
Earn XP
Description and Tags
not including equations
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
define energetics:
energy changes during chemical reactions
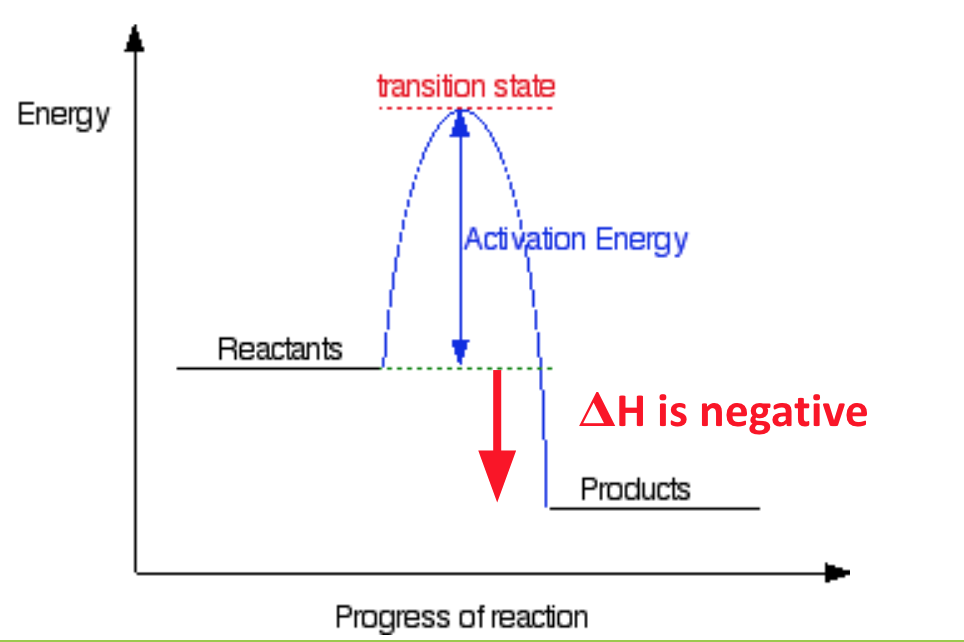
what is an exothermic reaction? describe the diagram for an exothermic reaction
a reaction in which heat energy is given off
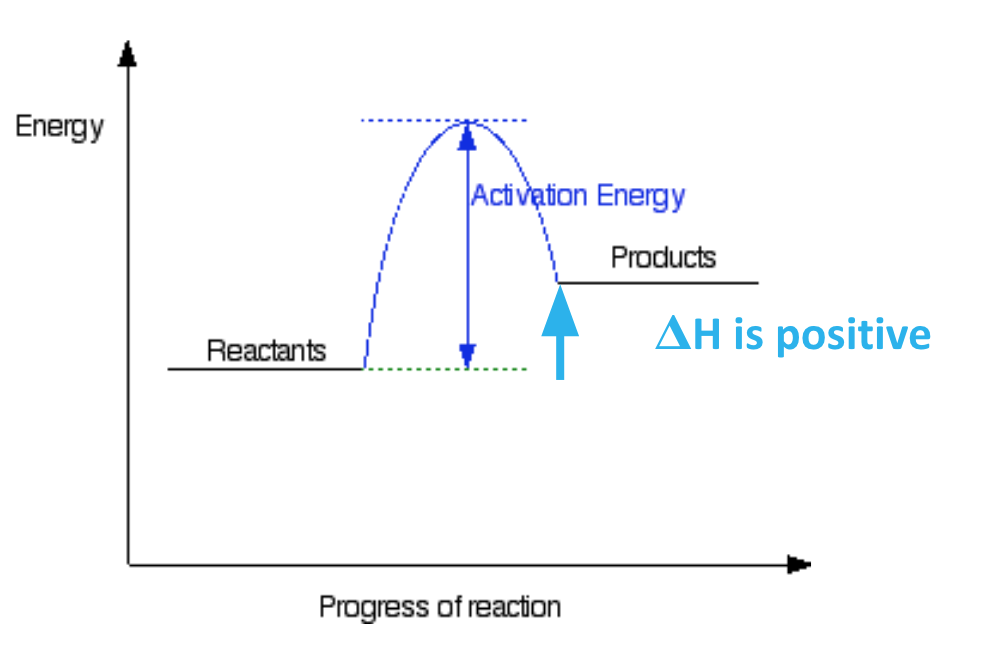
what is an endothermic reaction? describe the diagram for an endothermic reaction and state whether ΔH is +ve or -ve:
a reaction in which heat energy is taken in
what is enthalpy? what symbol is it represented by?
a measure of the heat content of a substance
represented by the symbol H
what are the units for enthalpy?
kJ mol-1
what is enthalpy change? what symbol(s) is it represented by?
heat energy change measured under conditions of constant pressure
represented by ΔH
what are standard conditions for a reaction?
100 kPa
stated temp (usually 298K)
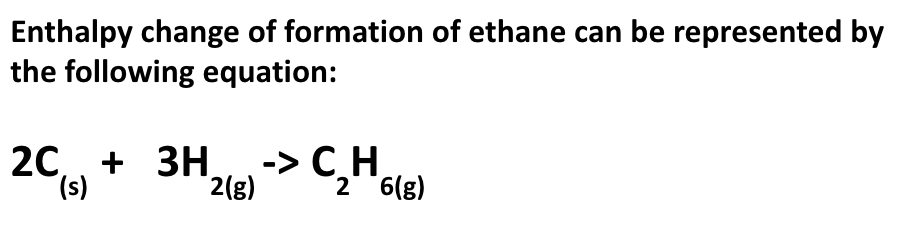
what is the standard enthalpy change of formation of a compound? how is it represented?
enthalpy change which occurs when:
1 mole of the compound is formed from its elements
under standard conditions and w/ everything in its standard state
represented by ∆f H Ɵ
what is the standard enthalpy change of combustion of a compound? how is it represented?
the enthalpy change which occurs when ONE MOLE of the compound is BURNED COMPLETELY in oxygen under STANDARD CONDITIONS and w/ everything in its STANDARD STATE
represented by ΔHƟc
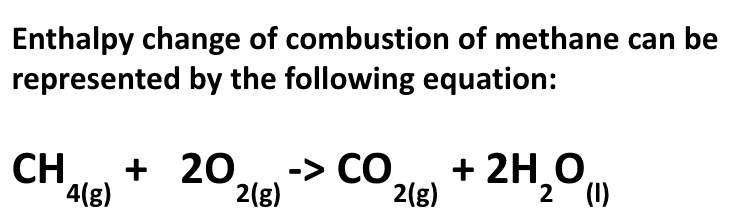
what is the specific heat capacity equation?
q = mcΔT
what is the equation for ΔH?
ΔH = q/moles
what is calorimetry?
the process of measuring the amount of heat energy released/absorbed during a chemical reaction