Purity, formulations and chromatography
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
What is a pure substance in chemistry?
A pure substance contains only one type of element or compound with no other substances mixed in.
How can you test the purity of a substance?
By measuring its melting or boiling point and comparing it to the known values of the pure substance.
How does the presence of impurities affect the melting and boiling points?
Impurities lower the melting point and increase the boiling point, often causing a range of temperatures rather than a sharp change.
What is a formulation?
A formulation is a mixture designed to produce a useful product with specific properties, made by mixing different substances in precise amounts.
Give three examples of formulations.
Paints, fuels, medicines, alloys, fertilisers, and cleaning agents.
Why are formulations important in the pharmaceutical industry?
The precise combination of active and inactive ingredients ensures the medicine is effective, safe, and delivers the correct dose.
What is chromatography used for?
To separate mixtures and identify substances based on their movement through a stationary phase.
What are the two phases in chromatography?
The mobile phase (solvent) and the stationary phase (paper or solid material).
How does chromatography work?
Substances move with the mobile phase at different rates due to differences in solubility and attraction to the stationary phase.
What is an Rf value?
The retention factor (Rf) is a ratio that measures how far a substance moves in chromatography relative to the solvent front.
How do you calculate the Rf value?
Rf=distance moved by substance/ distance moved by solvent
Why do different substances have different Rf values?
Because they have different solubilities in the solvent and attractions to the stationary phase.
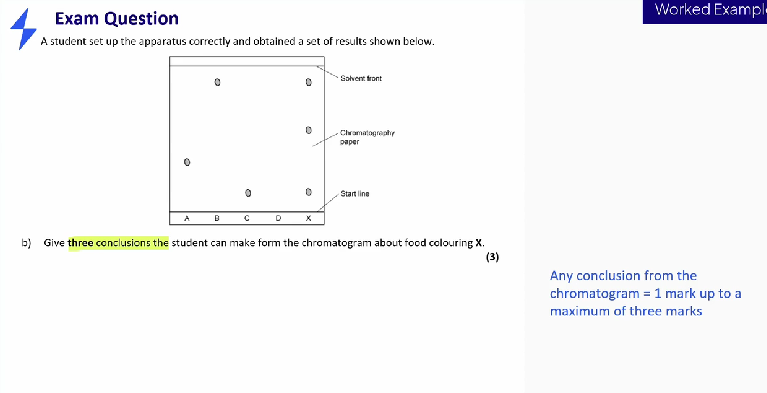
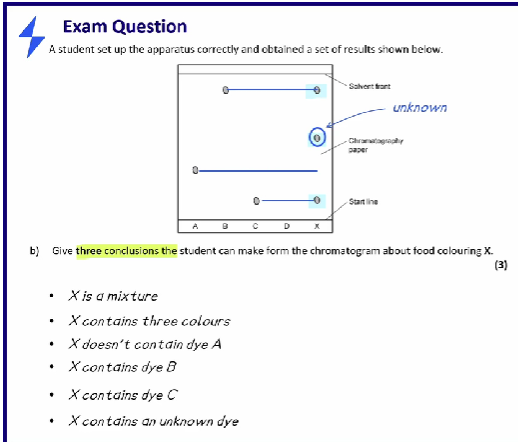
Give conclusions that can be made from this chromatogram
- X is a mixture
- X contains three colours
- X doesn’t contain dye A
- X contains dye B
- X contains dye C
Why must the starting line in paper chromatography be drawn in pencil?
Pencil does not dissolve in the solvent, unlike ink, which could interfere with the results.
describe the stationary phase
substance the molecules can’t move in so usually a solid. In this case it’s the paper so the substances bind to the paper. Substances less soluble are more attracted to the paper and so spend more time in the stationary phase so move up the paper slower
how to carry out chromatography
get a beaker, fill it with a shallow amount of solvent.
draw a pencil baseline on chromatography paper
place paper in beaker and mak sure the baseline remains above the solvent and they don’t submerge
place a lid on top so that the solvent doesn’t evaporate
wait for the solvent to seep up the paper and mark the solvent front
dry the chromatography paper
measure distance travelled by solvent so distance between start line and solvent front
measure distance between start line and spot to calculate distance travelled by the substance
describe the mobile phase
substances the molecule can move in eg. liquid or a gas, in this case it’s the solvent we use, the chemicals more soluble in the solvent spend more time in the mobile phase and so move up the paper faster