CHEM ENERGETICS
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
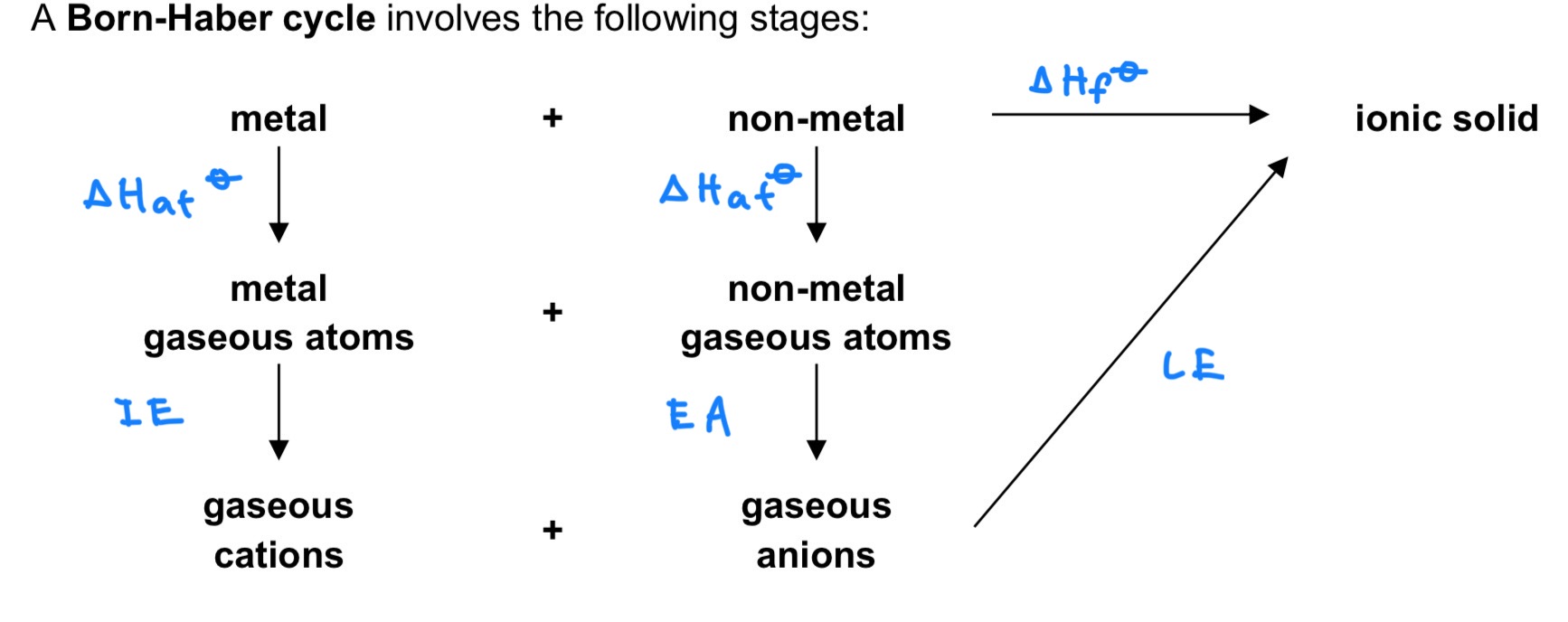
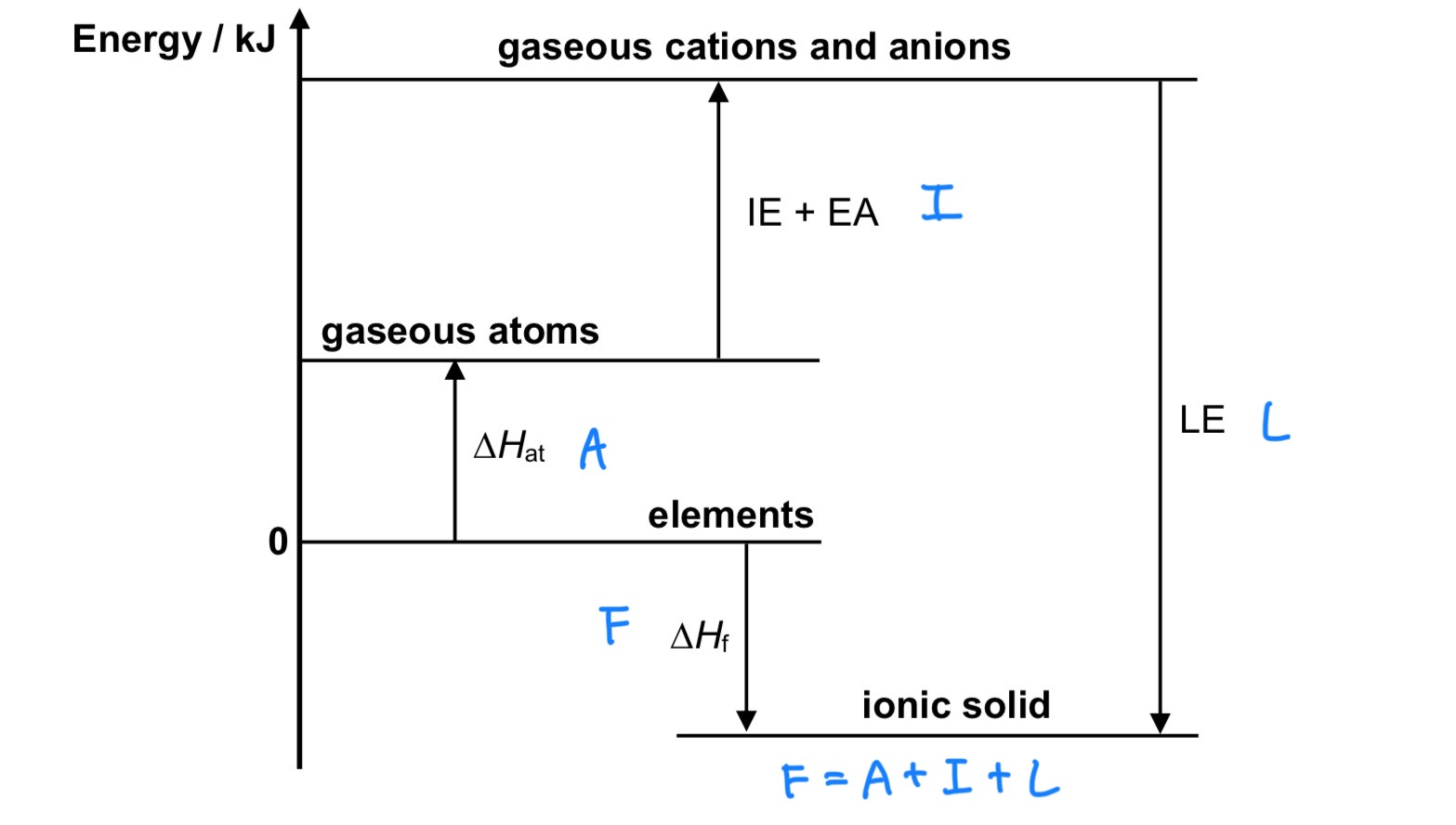
Define △H atomisation for elements and compounds. Is it exothermic or endothermic?
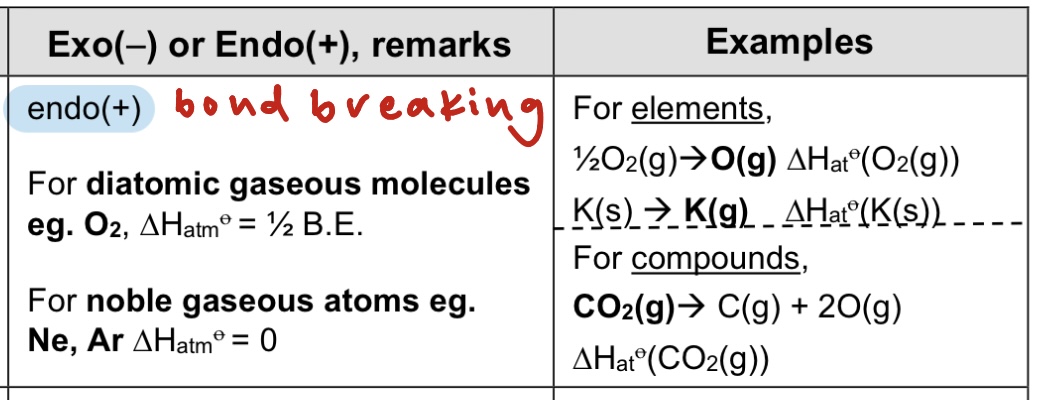
For elements, it is the energy required to form 1 mole of gaseous atoms from the element in its standard state under standard conditions, at a specified temperature, usually 298K.
For compounds, it is the energy required to form gaseous atoms from 1 mole of the compound in its standard state under standard conditions, at a specified temperature, usually 298K.
→ endo
Define BE (bond energy). Is it exothermic or endothermic?
It is the average energy required to break 1 mole of covalent bond between 2 atoms in gaseous state.
→ endothermic

Define △H combustion. Is it exothermic or endothermic?
It is the energy released when 1 mole of substance is completely burnt in excess oxygen under standard conditions, at a specified temperature, usually 298K.
→ exothermic
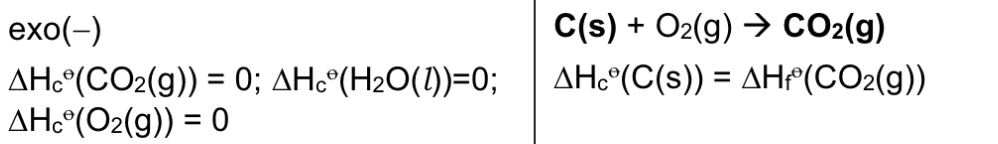
Define △H formation. Is it exothermic or endothermic?
It is the enthalpy change when 1 mole of substance is formed from its constituent elements in their standard states under standard conditions, at a specified temperature, usually 298K.
→ exo or endo (can be both)

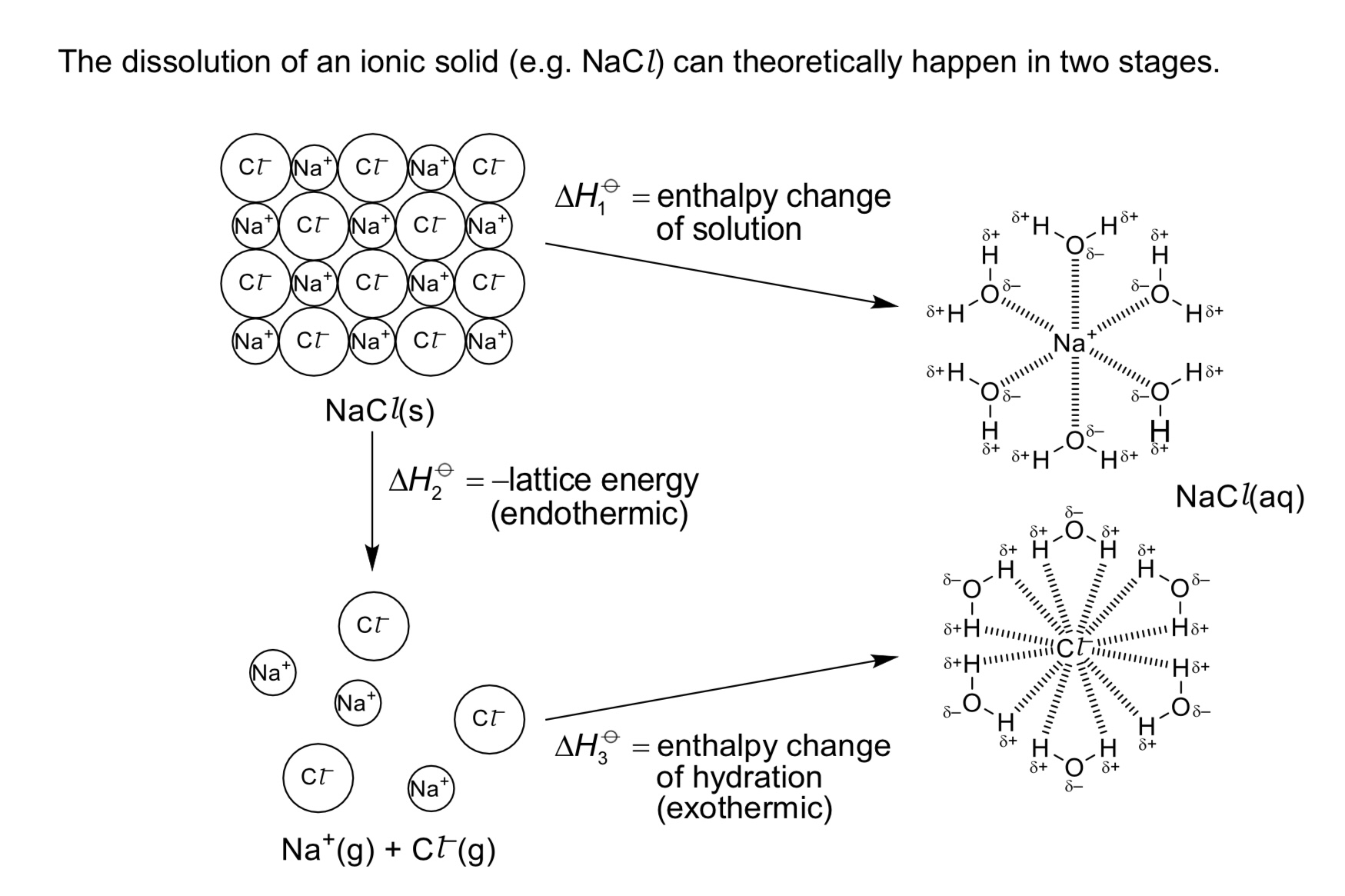
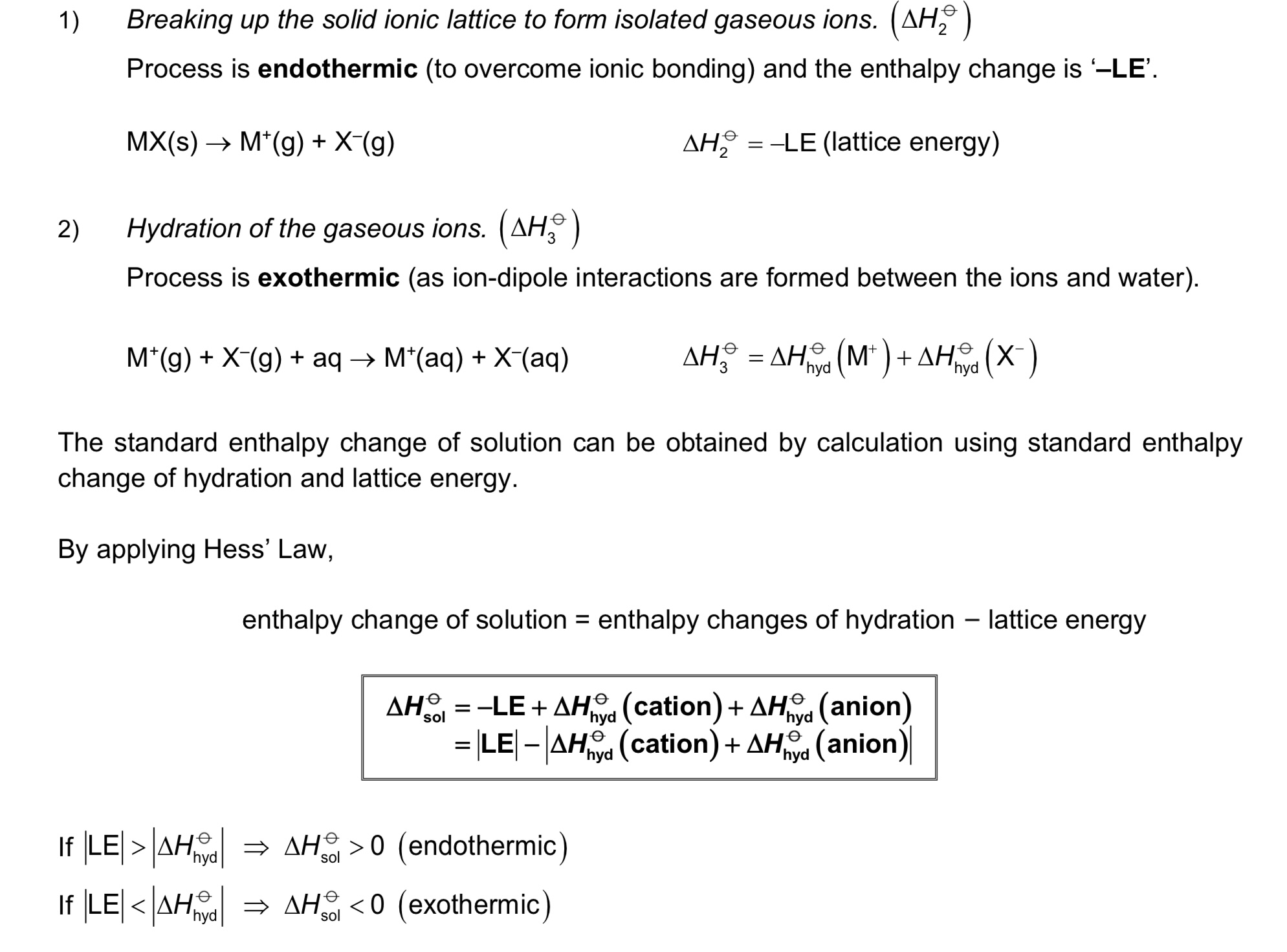
Define △H hydration. Is it exothermic or endothermic?
It is the energy released when 1 mole of free gaseous ions is hydrated under standard conditions, at a specified temperature, usually 298K.
→ exo
→ note that ion-dipole interactions between gaseous ions and polar water molecules are formed during hydration. the stronger the ion-dipole interactions, the more exothermic the △H hyd.
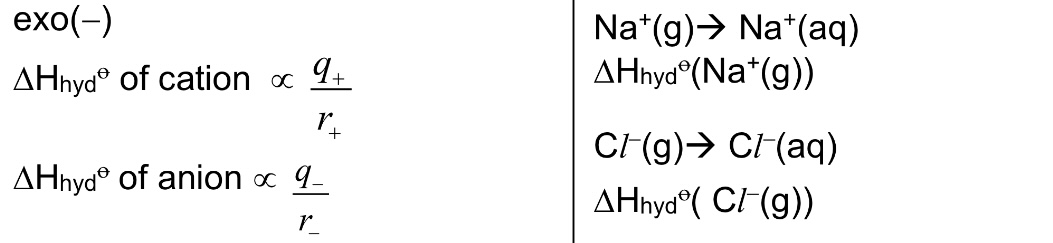
Define LE (lattice energy) / △H LE. Is it exothermic or endothermic?
It is the energy released when 1 mole of solid ionic compound is formed from its constituent gaseous ions
→ exo
→ note that generally, the larger the magnitude / numerical value of LE, the stronger the ionic bonds are
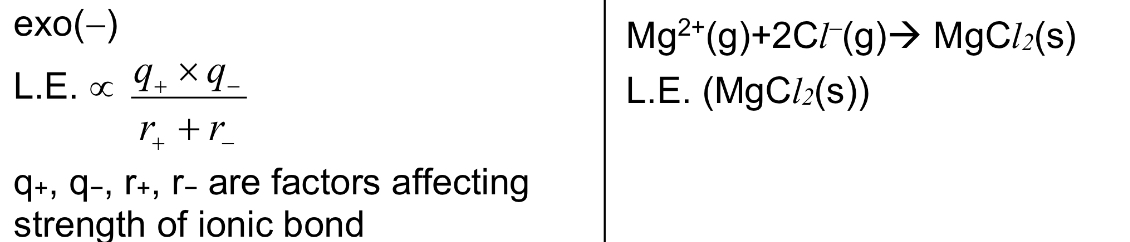
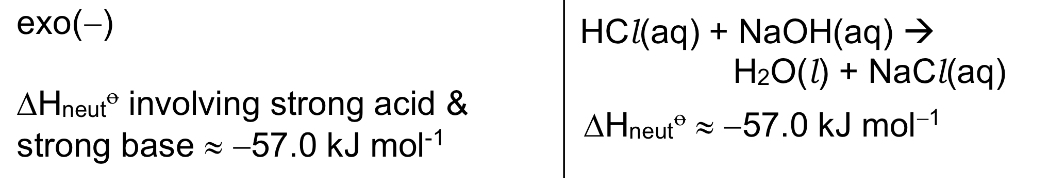
Define △H neutralisation. Is it exothermic or endothermic?
It is the energy released when 1 mole of water is formed in a neutralisation reaction between an acid and a base under standard conditions, at a specified temperature, usually 298K.
→ exo
→ note that △H neut involving weak acid or weak base may be less exo since energy is absorbed to dissociate/ionise the undissociated/unionised weak acid or weak base.
Define △H solution. Is it exothermic or endothermic?
It is the energy change when 1 mole of substance is completely dissolved in an infinite volume of solvent under standard conditions, at a specified temperature, usually 298K.
→ exo or endo (can be both)

Define 1st electron affinity (EA). Is it exothermic or endothermic?
It is the energy change when 1 mole of electrons is added to 1 mole of gaseous atoms to form 1 mole of singly charged gaseous anions.
→ exo or endo (can be both)
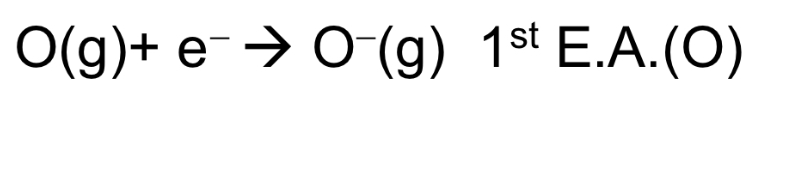
Define 1st ionisation energy (IE). Is it exothermic or endothermic?
It is the energy absorbed when 1 mole of electrons is removed from 1 mole of gaseous atoms to form 1 mole of singly charged gaseous cations.
→ endo
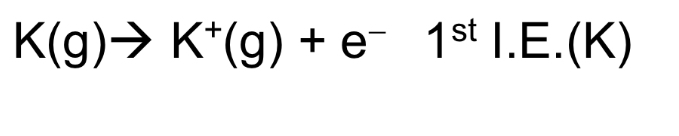
What is the equation / relationship between △H soln, △H hyd and △H LE?
△H soln = △H hyd - △H LE
Distinguish ionisation from dissociation (for understanding)
→ Dissociation is the separation of particles from an ionic compounds.
→ Ionisation is the process that produces new charged particles.
What conditions are standard?
→ pressure = 1 bar / 10^5 Pa
→ conc for solutions = 1 moldm^-3
→ any temp ok, but usually 298K / 25 °C
Define Hess’ Law.
Hess’ Law states that the enthalpy change of a reaction is determined only by the initial and final states and is independent of the reaction pathway taken.
Define q = -mc△T.
→ q is the heat change, in J
→ m is the mass of solution, in g
→ c is the specific heat capacity of water, at 4.18 or 4.2 J g^-1 K^-1
→ △T is the temperature change of solution, final temp - initial temp, in K or °C
Define q = -C△T.
→ q is the heat change, in J
→ C is the heat capacity of the solution, in J K^-1
→ △T is the temp change of the solution, final temp - initial temp, in K or °C
Define △H reaction = q/n
→ q is the heat change of reaction, = -mc△T or -C△T.
→ n is the amount of limiting reagent or specified substance
→ sign of △Hr (+ or -) denotes whether reaction is endothermic or exothermic
Calculate the efficiency of a combustion reaction.
q’ = x/100 q
where
→ q’ is heat absorbed by calorimeter (including its contents)
→ q is the total heat released from combustion
→ x is the efficiency of combustion reaction
What is the relationship between total BE of bonds broken and total BE of bonds formed?
△Hrxn = Σ (B.E. of bonds broken) − Σ (B.E. of bonds formed)
note: remember you need to break bonds of the reactants to form the bonds of the products
What is the relationship between Σn△Hf (products) and Σm△Hf (reactants)?
△Hrxn = Σn△Hf (products) - Σm△Hf (reactants)
note: remember acronym FPR, “fdr”
What is the relationship between Σn△Hc (products) and Σm△Hc (reactants)?
△Hrxn = Σn△Hc (reactants) - Σm△Hc (products)
note: remember acronym CRP, “crap”
Why may △Hr differ from actual △H?
1) If BE was used to calculate △Hr, BE are average values and may not be accurate
2) Heat loss to / gain from surroundings was not taken into account
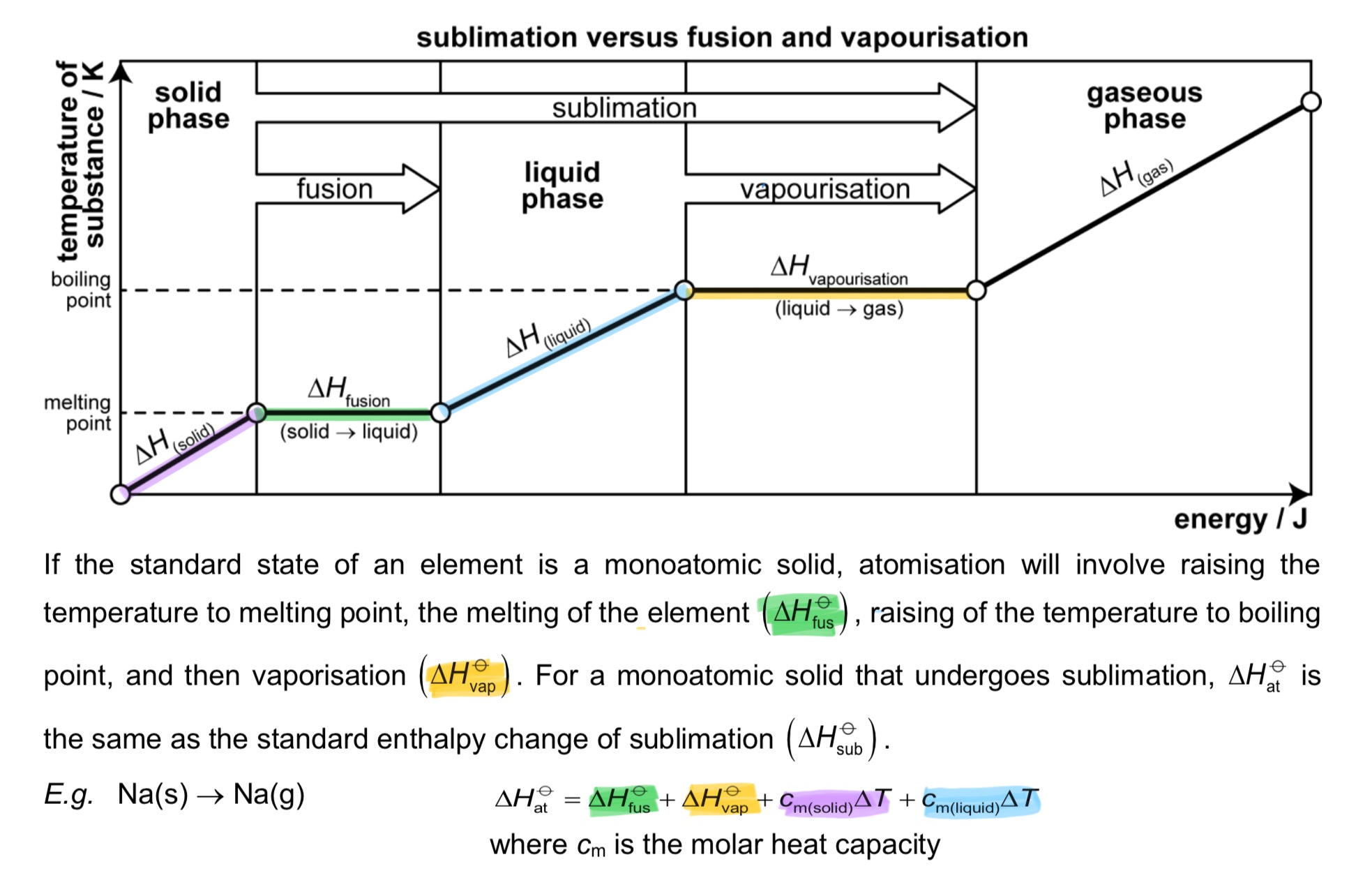
If the standard state of an element is a monoatomic solid, atomisation will involve raising the temperature to melting point, the melting of the element (△H fusion), raising of the temperature to boiling point, and then vaporisation (△H vaporisation).
For a monoatomic solid that undergoes sublimation, △H atomisation is the same as the standard enthalpy change of sublimation (△H sublimation.
→ E.g. Na(s) → Na(g)
→ △H at = △H fus + △H vap + Cm (solid)△T + Cm (liquid)△T, where Cm is the molar heat capacity
△H at vs △H vap
→ △H vap does not break any covalent bonds, only overcomes IMF attraction to bring an element/compound from liquid to gaseous state
→ △H at breaks covalent bonds to form gaseous atoms
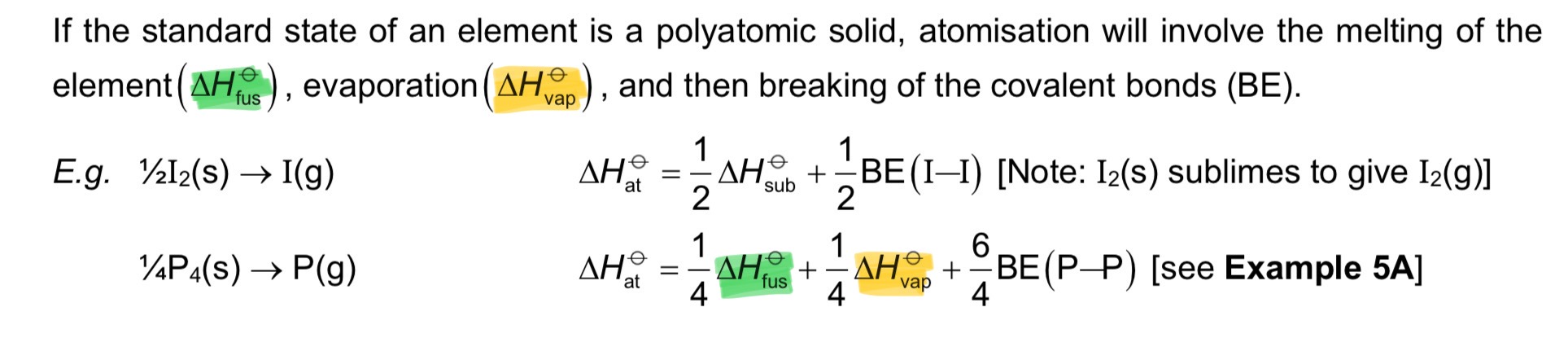
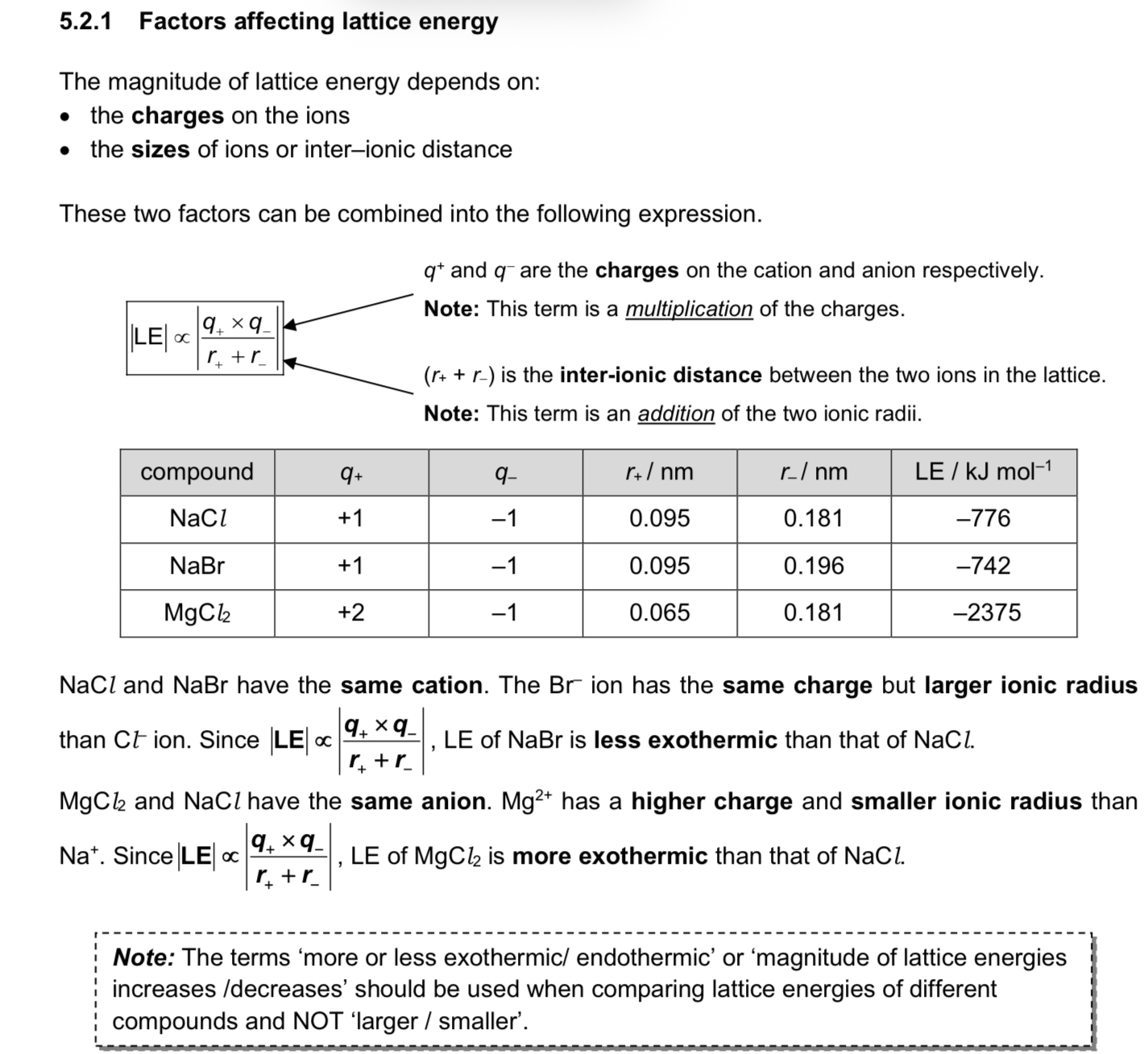
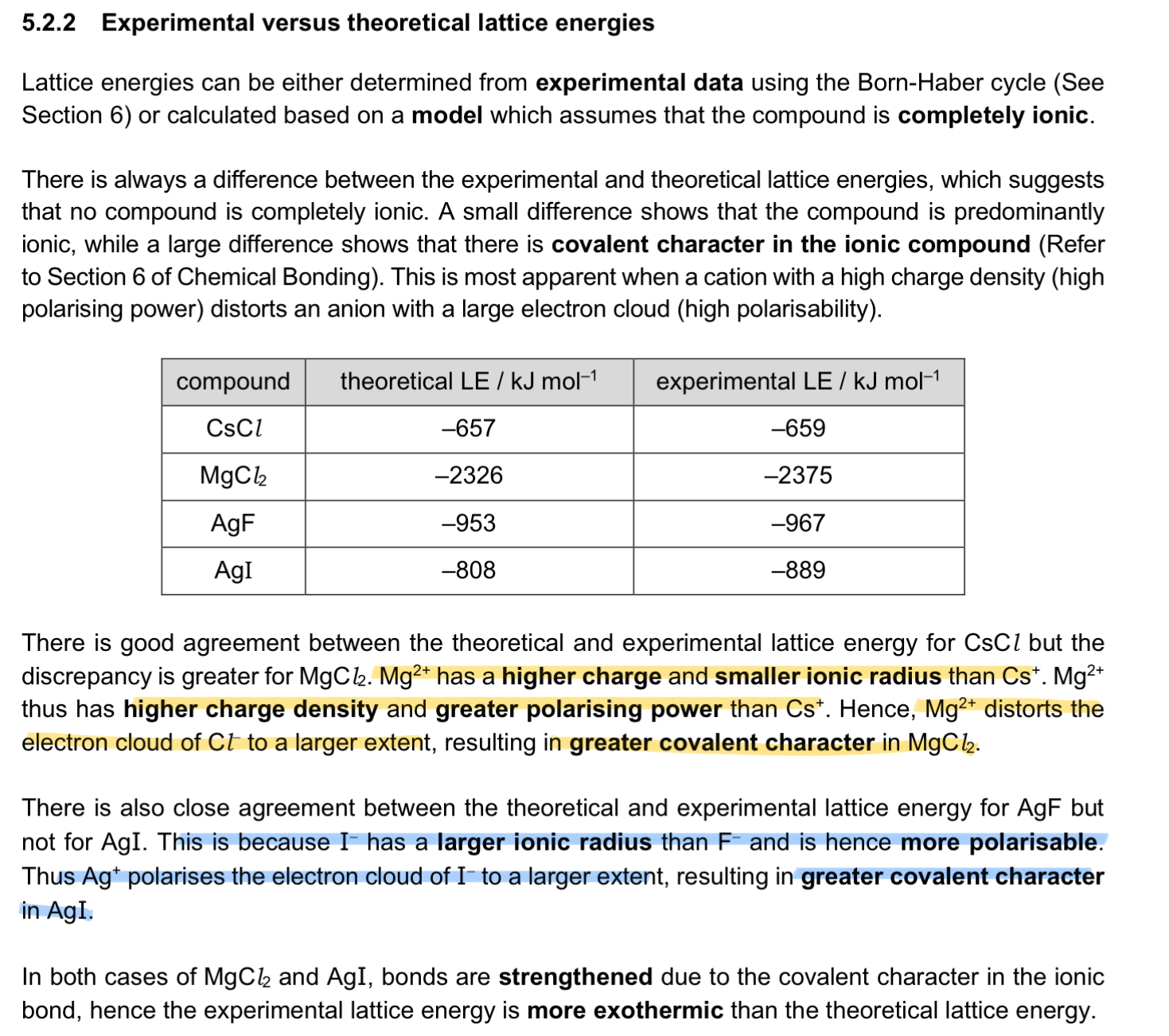
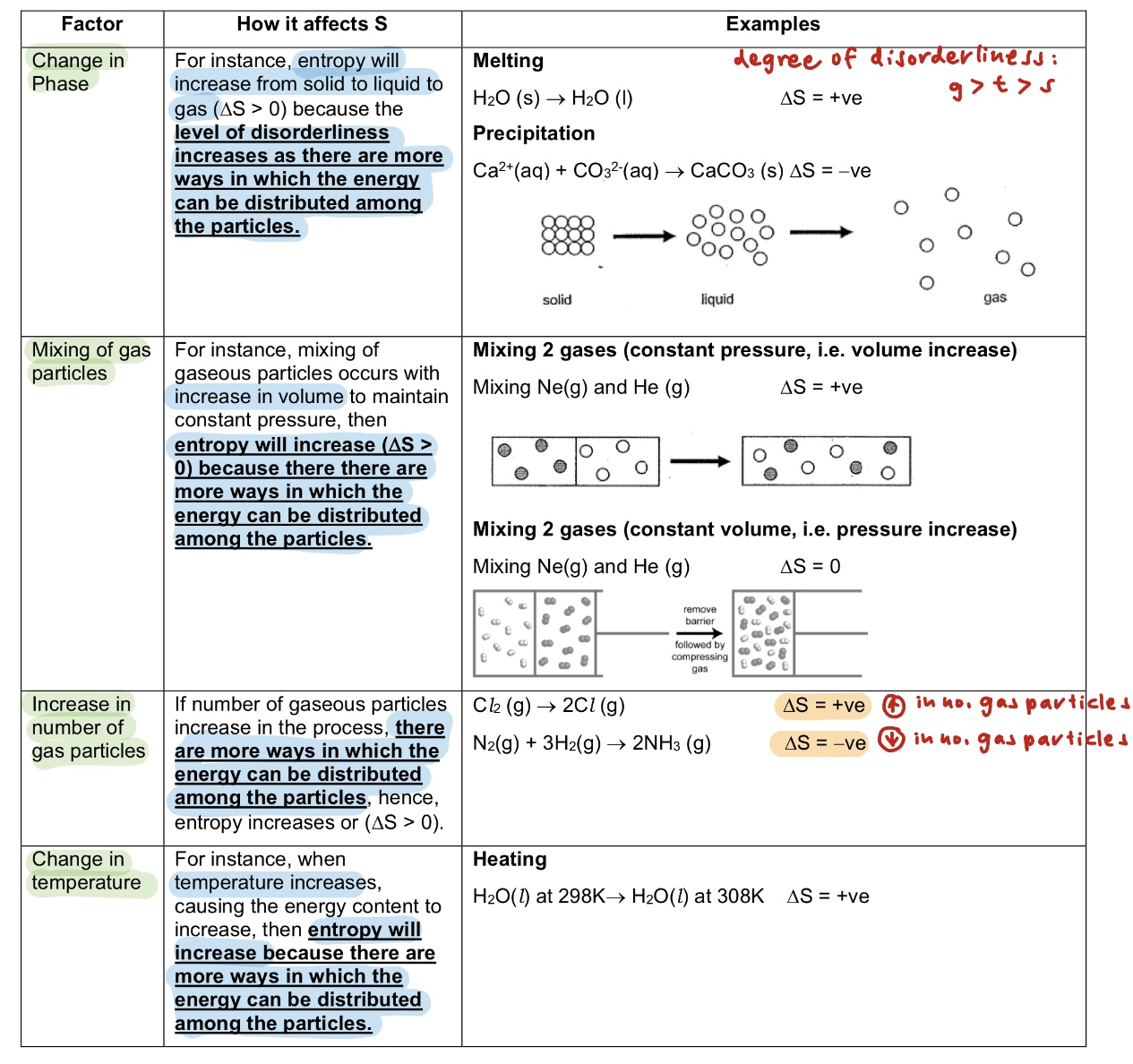
Define entropy.
Entropy is a measure of the level of disorderliness or randomness in a system.
List the factors that affect entropy.
What is the Gibbs fire energy equation and what they represent?
→ △G = △H - T△S
where
→ △G < 0 indicates spontaneous change, △G = 0 indicates dynamic equilibrium, △G > 0 indicates non-spontaneous change , in kJ mol^-1
→ △H is the enthalpy change, in kJ mol^-1
→ T is temperature, in K
→ △S is entropy change, in kJ mol^-1 K^-1
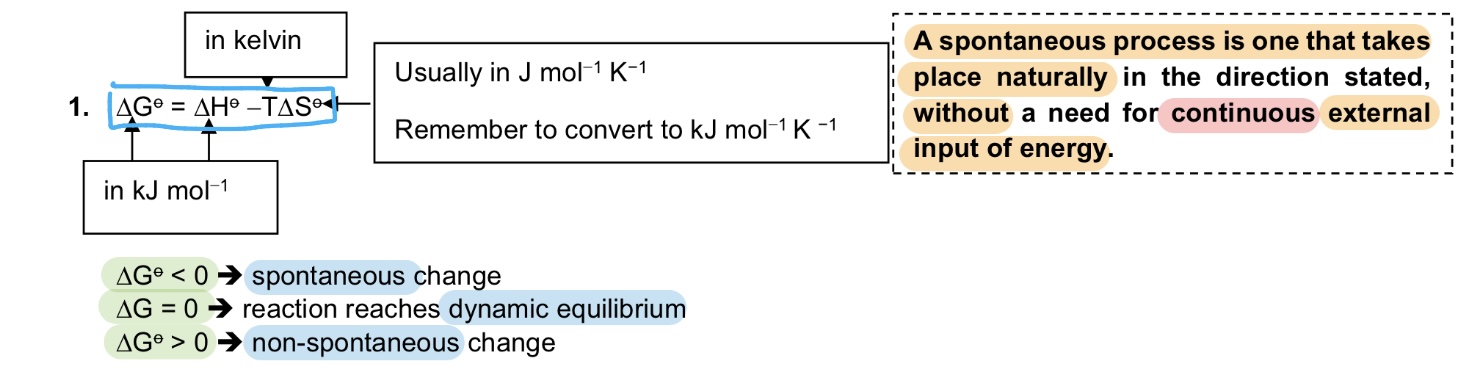
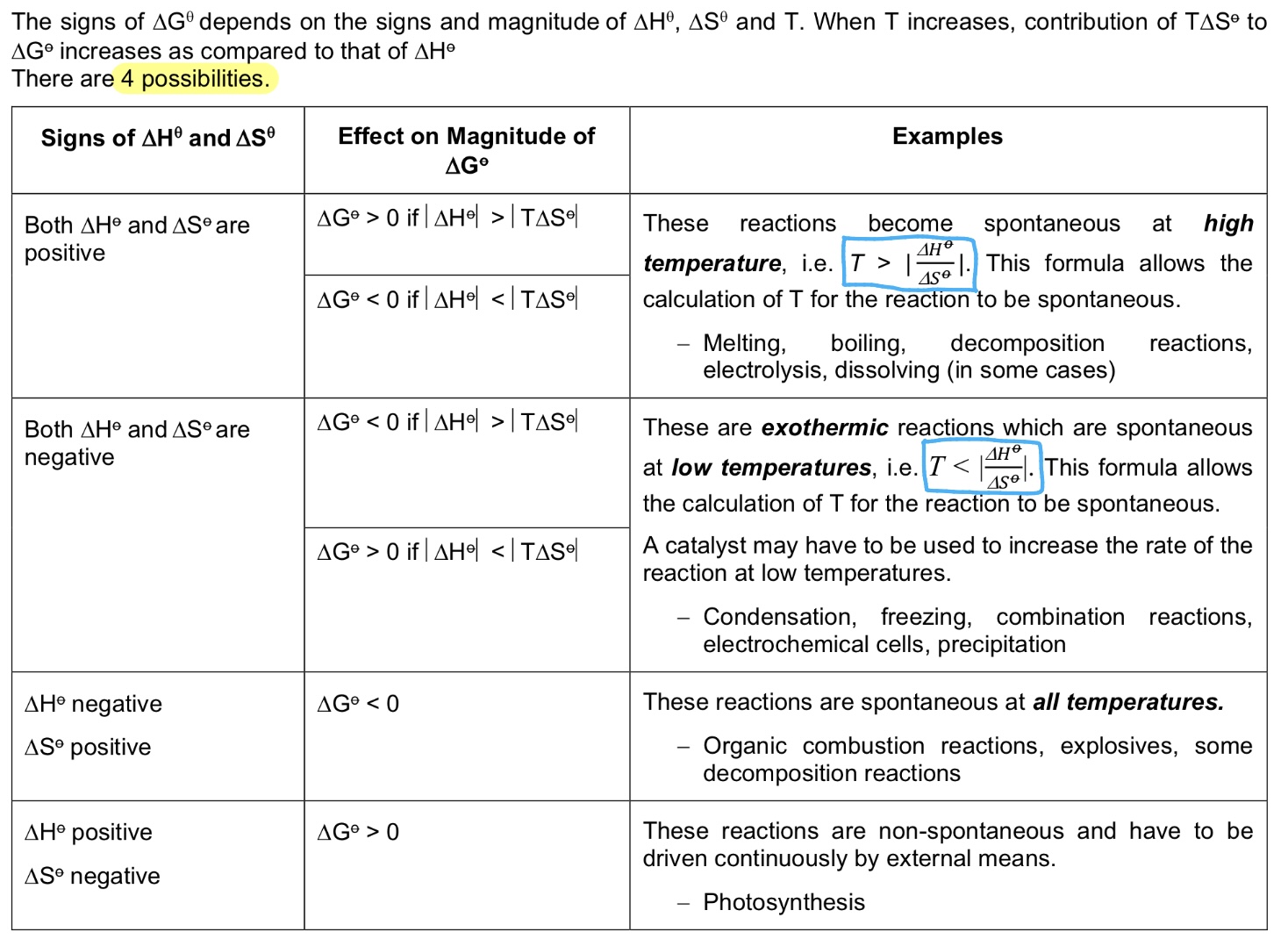
Explain the effect of temperature on △G