Anaerobic/Aerobic respiration 2
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
25 Terms
In the context of thermoregulation, when does metabolic rate increase in response to environmental temperature?
Metabolic rate increases below the Lower Critical Temperature (LCT) or above the Upper Critical Temperature (UCT).
Below LCT: ↑ metabolism generates heat to maintain body temperature.
Above UCT: ↑ metabolism supports cooling mechanisms (e.g., panting, sweating) and prevents hyperthermia.
What are the two ways mammals regulate their temperatures?
Shivering thermogenesis
Ambient temperature is detected in peripheral receptors, e.g. in skin
Core temperature is detected by central sensors in hypothalamus
Signals integrated in DORSOMEDIAL HYPOTHALAMUS
Non-shivering thermogenesis (NST)
Cold is sensed by brain, activates the sympathetic nerves, releasing Noradrenaline, which is sensed by B-AR receptors found within brown adipocytes (Lipid droplets with mitochondria)
Activates cascade pathway: releases cAMP, Protein kinase A
Acute Effects
• stimulation of lipolysis
• activation of UCP-1 activity
Chronic Effects
• UCP-1 gene transcription
• mitochondrial biogenesis
• hyperplasia of brown adipose tissue
• recruitment of brown adipocytes in white adipose tissue depots
What is shivering thermogenesis?
Involuntary somatic motor response to increase body temperature
Muscle contraction of antagonistic muscles
Contraction but little net movement
Produces heat through hydrolysis of ATP
Metabolically expensive and relatively inefficient
What is the role of UCP1 in non-shivering thermogenesis?
Uncoupling protein 1 (UCP1) in inner mitochondrial membrane
UCP1 (Uncoupling Protein 1) is found in brown adipose tissue and allows protons to bypass ATP synthase in the mitochondrial inner membrane.
This “uncouples” oxidative phosphorylation: electron transport continues, but ATP is not produced.
Protons bypass ATP synthase and leak back across the inner mitochondrial membrane (IMM) via UCP1.
Energy from the proton gradient is released as heat instead of making ATP, warming the body without muscle shivering.
Important in cold adaptation, especially in neonates and small mammals.
Only get production of heat (no ATP)
What physiological process is non-shivering thermogenesis used?
Hibernation
Abandon euthermia for seasonal heterothermy
“Abandon euthermia for seasonal heterothermy” means the animal stops maintaining a constant body temperature and allows it to drop during certain seasons (e.g., winter) to conserve energy.
Triggered by low ambient temp, food deprivation, short days
Animals reliant on food caches/body reserves over weeks or months
What happens to core body temperature during hibernation?
What other parameters are also effected?
Core Tb follows ambient and can get close to 0C
Respiration rate, HR and metabolic rate all drop
Why do animals have to arouse through hibernation and why is it important?
Arousal is energetically expensive, so why bother?
Periodic arousal during hibernation restores body temperature, clears metabolic waste, maintains immune function, and allows essential physiological processes to continue.
Drink/eat
Waste removal- urine, urea
Cellular repair and protestasis
Activate a dormant immune system
Counter a 'sleep debt', neurogenesis
How do animals prepare for hibernation?
Acclimation to cold and preparation for hibernation
Must Increase brown-fat depots and UCP1 levels
What is the importance of NST in neonates?
At birth, profound thermolysis, huge loss in body temperature after birth
~38.8°C in utero to Ta
Peri/Post-natal catecholamine surge after birth which releases:
Norepinephrine, epinephrine, dopamine
These activate B-AR receptors which induce or increase NST
How has NST be used to treat obesity?
Chronic treatment with B3-AR agonist (ICI D7114) - which helps activate BAT receptors - increases non-shivering thermogenesis
Thermogenic and anti-obesity properties, reduces white fat and increases metabolic rate
Reduction in BW and girth
Stimulated metabolic rate
Led to appearance of BAT depots
Species specificity?
Tt not as effective in sheep.
Are pigs able to perform NST?
Pigs lack functional UCP1 protein- meaning they cannot perform non-shivering thermogenesis
Making them prone to neonatal hypothermia
But they have UCP-1 independent mechanisms
UCP-3 - in skeletal muscle, greater proton leakage, similar to NST
SERCA thermogenesis
What is SERCA thermogenesis?
Sarco(endo) plasmic reticulum (SR) Ca2+-ATPase (SERCA)
Muscle-based non-shivering thermogenesis
SERCA's Role: SERCA pumps normally use ATP to shuttle Ca2+ from the muscle cell's cytoplasm back into the sarcoplasmic reticulum (SR) for relaxation.
Sarcolipin (SLN) Binding: The small protein sarcolipin binds to SERCA, preventing efficient Ca2+ transport.
Futile Cycling: This binding uncouples Ca2+ transport from ATP use, meaning ATP is still broken down (hydrolyzed), but the Ca2+ "slips" back out, forcing the pump to work harder, generating heat without performing its primary relaxation job.
Heat Production: This constant, inefficient cycling (futile cycling) releases a significant amount of energy as heat, contributing to thermogenesis (heat generation).
What condition are some pigs predisposed to that may alter their thermoregulatory abilities?
Inherited mutation on Ryanodine receptor
Massive build up of Calcium in muscle
Channel opens excessively → continuous Ca²⁺ release from SR
Cytoplasmic Ca²⁺ rises uncontrollably
Consequences:
Sustained muscle contraction (rigidity)
Excess ATP consumption by SERCA
Ca²⁺ must be pumped back into SR → uses ATP → generates heat
Rapid hyperthermia (life-threatening)
Metabolic acidosis and rhabdomyolysis
Stress, exercise, heat, volatile anaesthetics e.g. Halothane, can induct malignant hyperthermia
How does aging affect physiological function?
Age-dependent decline in instrinsic physiological function
Increase in age-specific mortality
Decrease in age-specific reproductive rate, more profound in females than males (As it is more energetically demanding)
How does the aging rate vary between or within species?
Aging rate between or within species varies
Seems increase in body mass = increase longevity
As opposed to small animals = decreased longevity
But there are outliers
And variation WITHIN species - large dogs live shorter lives than small dogs generally speaking
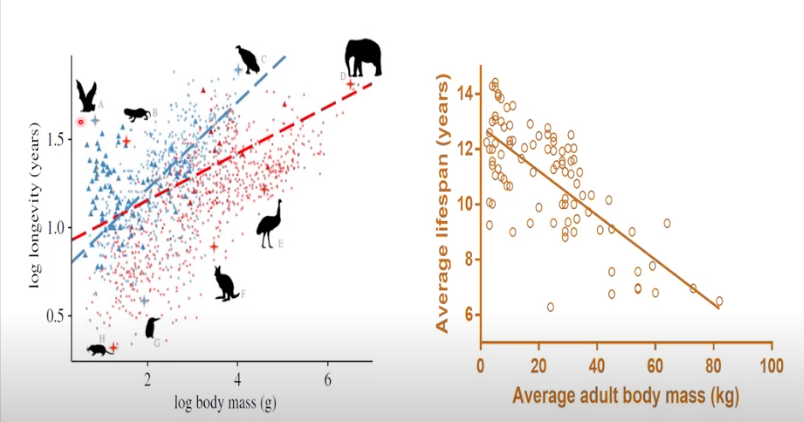
What does “Aging is conserved” mean?
“Aging is conserved” means that the fundamental biological mechanisms of aging are similar across different species.
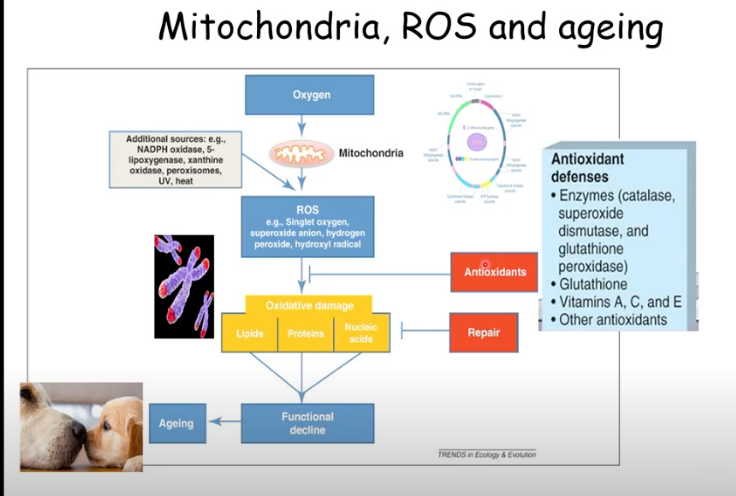
What are the important processes ROS are involved in?
ROS essential for immune function, cell growth/adaptation, cell signalling
BUT - also involved in aging
Mitochondria produce ROS (e.a. Singlet oxygen, superoxide anion. hydrogen peroxide, hydroxyl radical)
Causing oxidative damage → leads to functional decline associated with aging
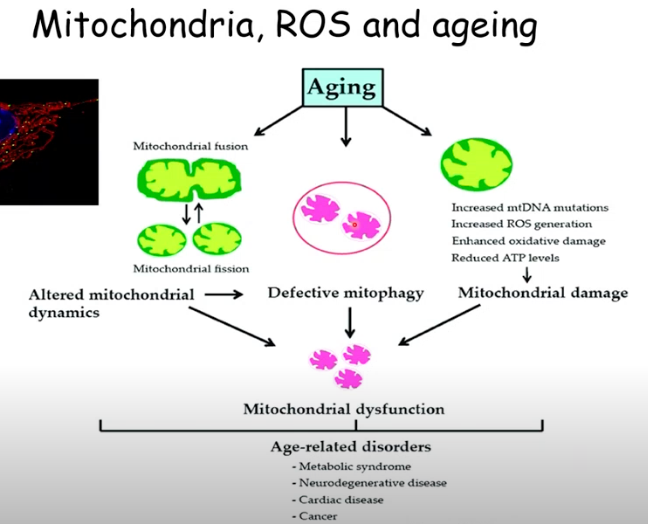
How does aging affect mitochondria?
What are inherited metabolic diseases?
Definition: Genetic disorders caused by rare defects in enzymes or proteins in biochemical/metabolic pathways.
Link to Mendelian inheritance: These diseases often follow autosomal recessive, dominant, or X-linked patterns, explaining why some family members are affected and others are carriers.
Phenotypic effects: Metabolic defects lead to accumulation of toxic substances or failure to produce essential metabolites, resulting in classic “failure to thrive” phenotypes, developmental delay, or organ dysfunction.
What are the main features of Alkaptonuria?
“Black Urine Disease” due to oxidation of Homogentisic acid
Mutation in homogentisate 1,2 dioxygenase (HMG) gene
Inability to metabolise tyrosine and phenylalanine, causing a build-up of Homogentisic acid- oxidises to pigment (ochre build up in sclera of eyes and urine)
Ochronotic arthritis - calcification in joints
Calcification of invertebral discs
Kidney stones
Valve stenosis
What are the main features of Fanconi syndrome?
Variety of underlying causes, can be inherited, acquired, or caused by exogenous factors
Causes proximal kidney tubule dysfunction
Thus: Urinary loss of electrolytes and nutrients
Growth retardation
Diarrhoea
Hypokalaemia
Hyponatraemia
Hypochloraemia
A single gene mutation in the gene that encodes Enoyl-CoA may cause what syndrome?
Not getting reduction of Acetyl-CoA (Which is crucial for Krebs cycle to produce the electron donors)
Thus: Impaired fatty acid oxidation, mitochondrial dysfunction and impaired functionality of proximal kidney tubule.....lactate acidosis
Fanconi Syndrome
What is MELAS (Mitochondrial Encephalopathy Lactic Acidosis and Stroke-like Episodes)?
Clinical signs in humans usually between 2-15 years
Mutation in tRNA gene MT-TL1 causes ~80% of all cases
tRNALeu involved in assembly of OXPHOS Complex 1 (Found in IMM, involved in oxidative phosphorylation)
Seizures, recurrent headaches, vomiting, hemiparesis, vision and hearing loss, loss of motor skills, cardiomyopathy and intellectual disability
What is Maple Syrup Urine Disease?
Inherited aminoacidopathy
Dysfunction in mitochondrial branch-chain keto acid dehydrogenase (BCKDH)
Preventing proper breakdown of branched-chain amino acids, leading to accumulation of BCAAs and their keto acids, which are toxic and excreted in urine, giving the characteristic sweet smell.
Leading to build-up of leucine, valine and isoleucine- BCAA
Damage to mitochondrial DNA, reduced mitochondrial metabolism, apoptosis
Urine smells of maple syrup (in humans)
More common in Hereford cattle
Clinical signs within 2-4 days of age
Dullness, recumbency and opisthotonos
CNS dysfunction if not treated
What does “inborn error or metabolism” mean?
Inborn errors of metabolism (IEMs) are genetic disorders present from birth that cause defects in enzymes or proteins involved in metabolism, leading to abnormal buildup or deficiency of metabolic products.
Phenotype — often presents as “failure to thrive,” developmental delay, or organ dysfunction in newborns.