Types of Matter
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Atom
The smallest unit of matter.
Element
Contains the same type of atom.
Pure substance
Contains only one type of particle.
Elements and Compounds
2 types of Pure substances
Mixtures
Can be separated by physical changes because they are not chemically combined.
Homogeneous Mixture
Substances mixed evenly and look the same
Heterogeneous Mixture
Substances mixed, but you can see the different substances.
Sugar water, lemonade, tea
Examples of Homogeneous mixtures
Fruit salad, soup
Examples of Heterogeneous mixtures
Molecule
When two or more atoms bond together
Compound
Two or more different atoms bonded together
Examples of compounds
H20, CO2, NH3
Examples of elements
O2, N, Fe, Ag
Mixture of Elements
.
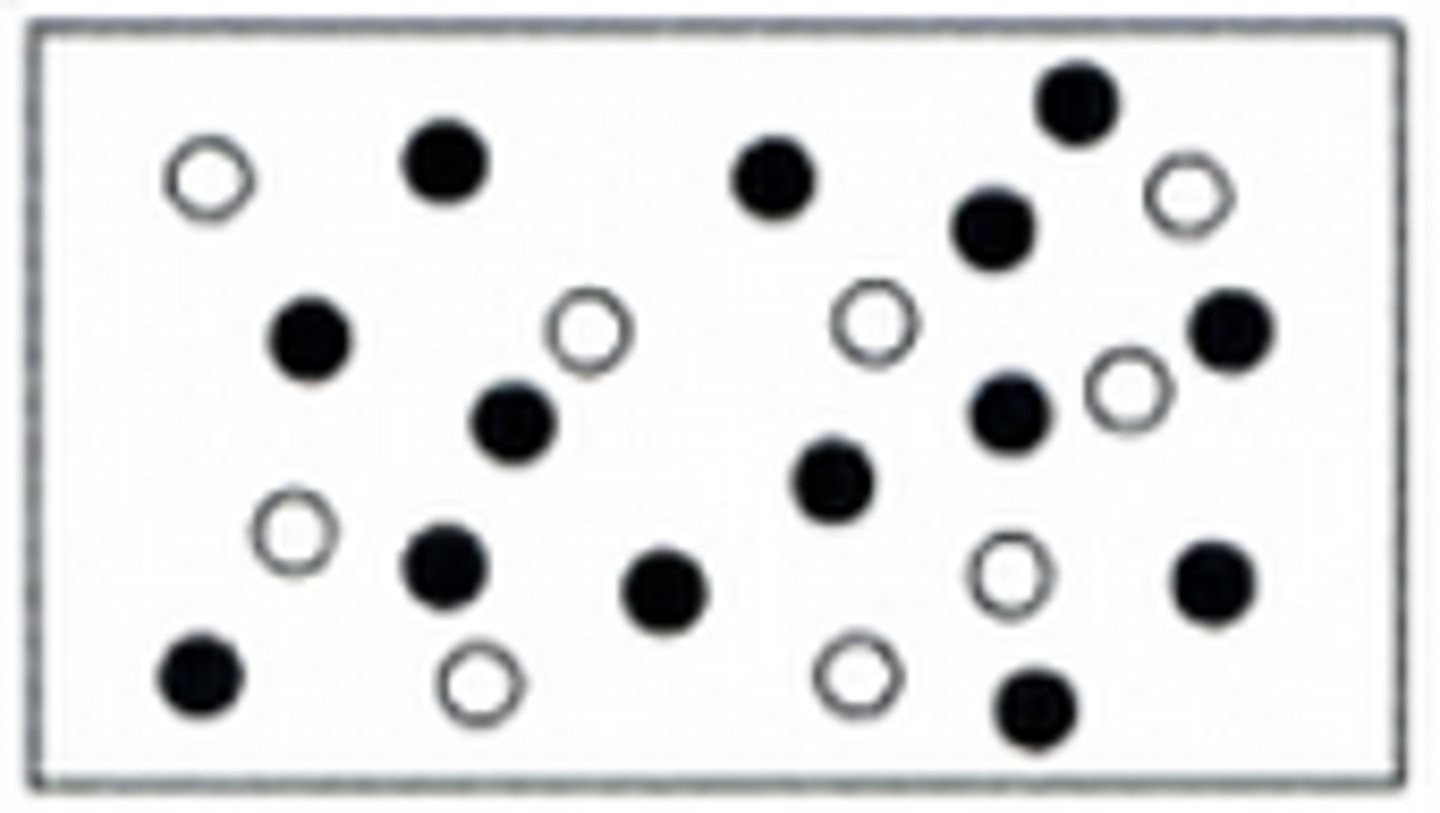
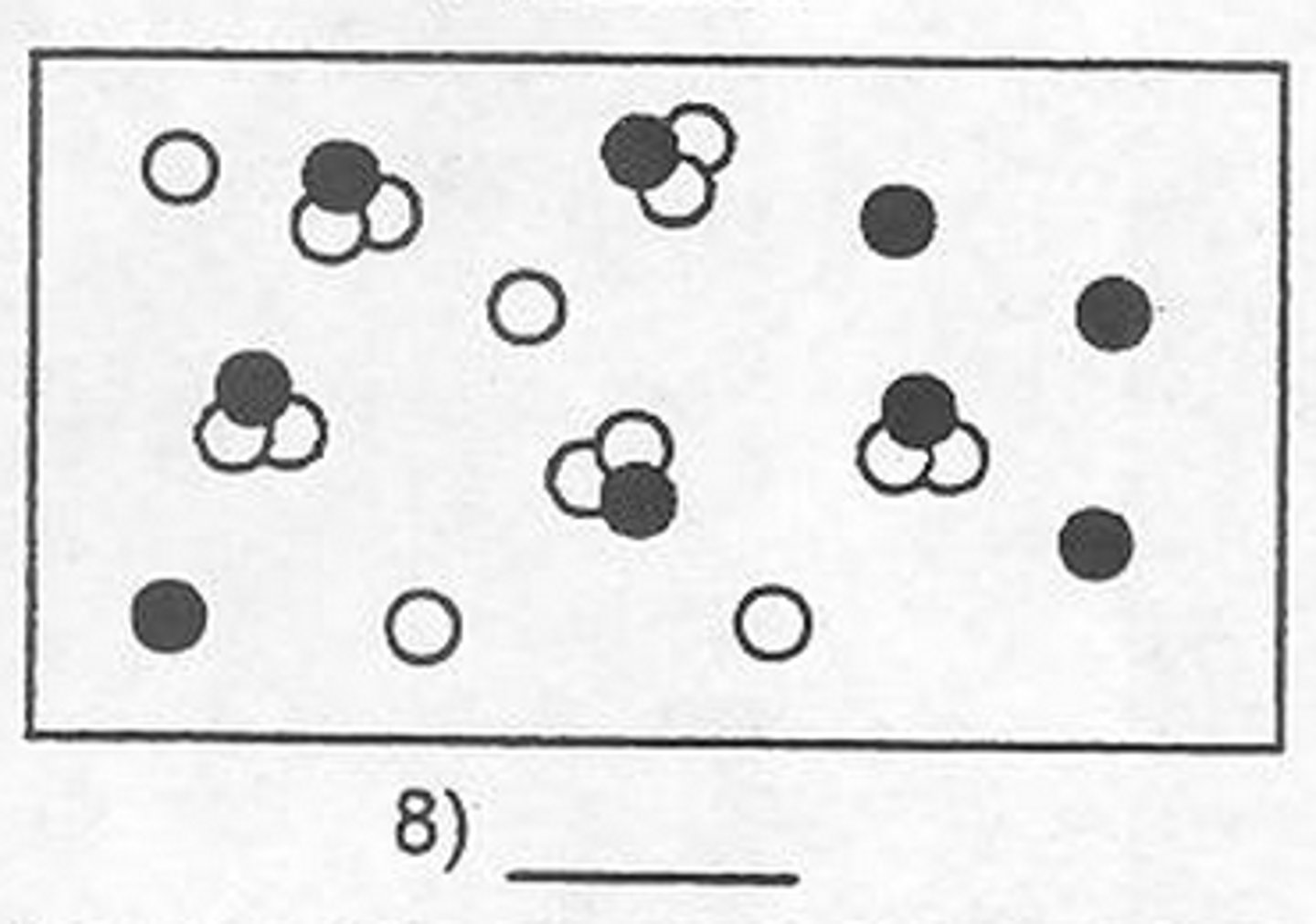
Mixture of Compounds
.
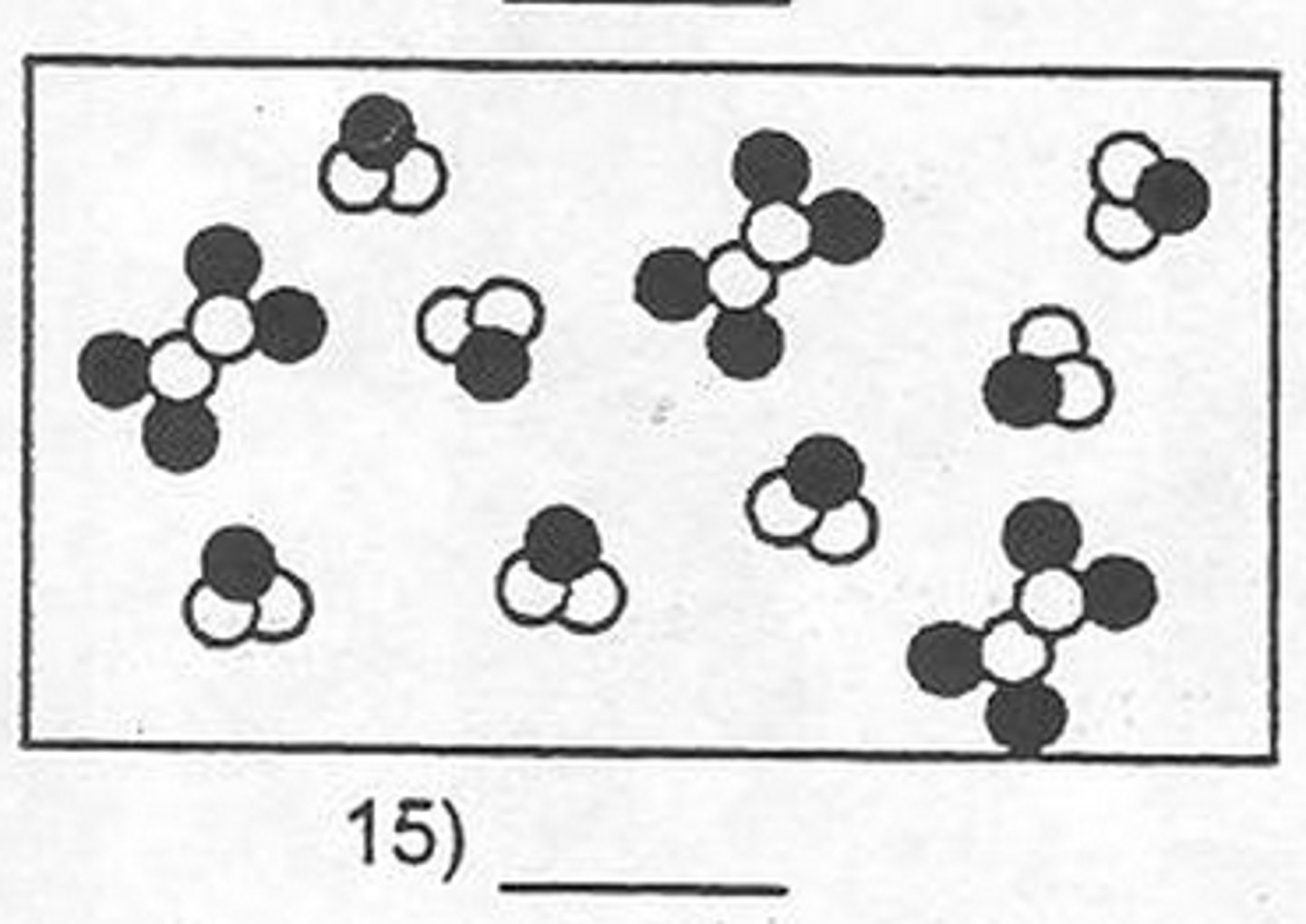
Mixture of Elements and Compounds
.
6 (six)
The number of atoms of Carbon in C6H12O6
1 (one)
Pure substances are made of only _________ type of particle
Types of physical changes to separate mixtures
evaporation, filtration, magnetic attraction
Molecule Element
2 or more of the same type of atom bonded together