2.1: Models of bonding and structure
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Ionic compounds
a chemical compound consisting of positively charged ions (cations) and negatively charged ions (anions) held together by strong electrostatic forces
why high melting point
build up into a strong lattice. Ionic compounds have high melting points as considerable energy is required to overcome these forces of attraction.
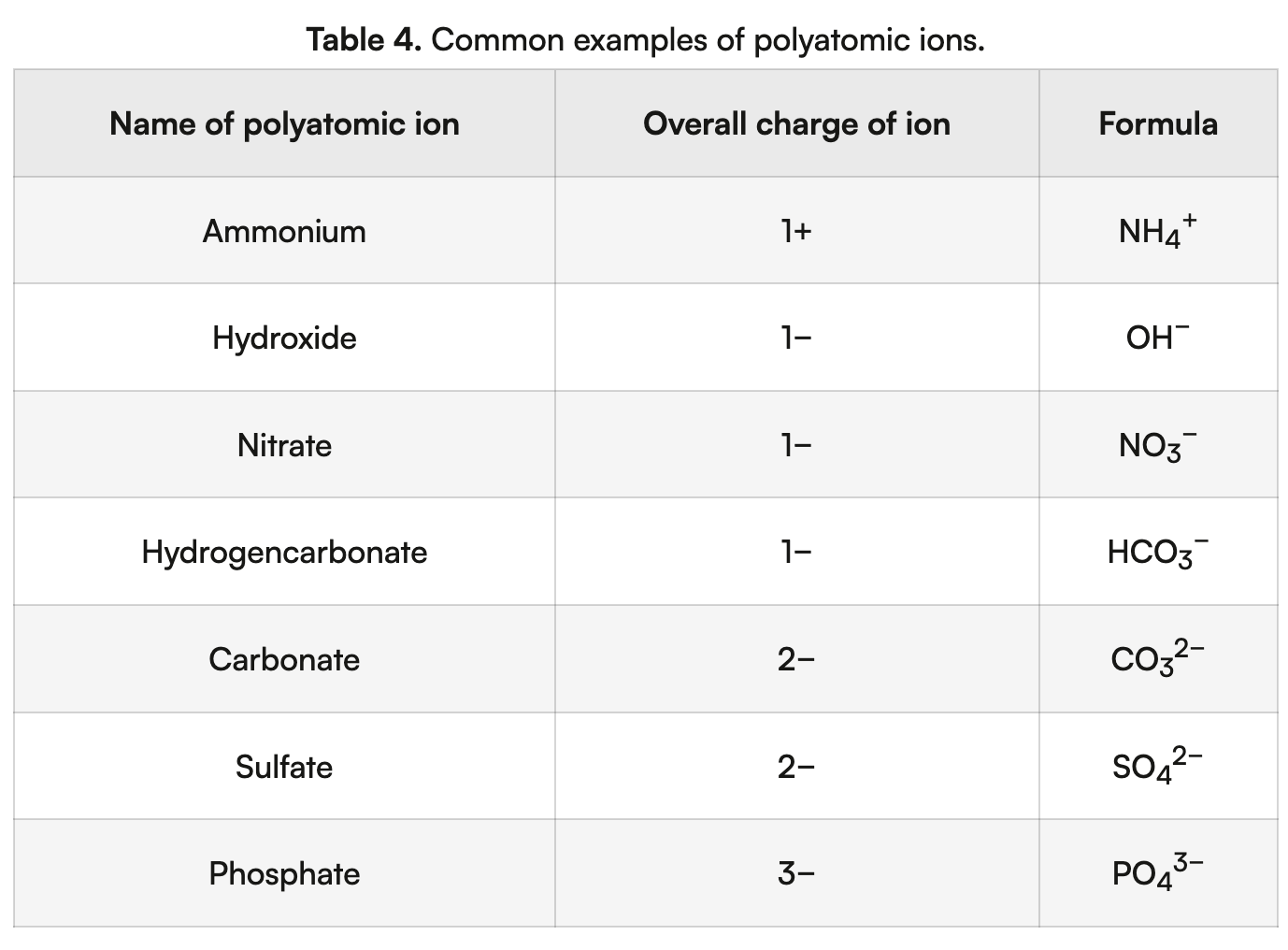
polyatomic ions
ions composed of two or more atoms that are covalently bonded together and carry an overall electrical charge, where the charge is often spread (delocalized) over the whole ion
Lattice
A three-dimensional regular repeating arrangement of ions, atoms or molecules in a crystalline solid.
lattice enthalpy
enthalpy change that occurs when one mole of an ionic compound is broken apart into its constituent gaseous ions under standard conditions.
hydration enthalpy
enthalpy change that occurs when one mole of gaseous ions dissolves in sufficient water to form an infinitely dilute aqueous solution under standard conditions.
are ionic compounds conductive
when solid no. when molten yes (ions free to move) where they are chemically
decomposed at the respective electrodes.
Covalent bonding
Covalent bonding involves the sharing of one or more pairs of electrons so that each atom in the molecule achieves a noble gas configuration. electrons are shared and attracted electrostatically by positive nuclei, resulting in a directional bond between the atoms to form a molecule.
octet rule
atoms tend to form chemical bonds in such a way that each atom has eight electrons in its outermost electron shell, giving it the same electron configuration as a noble gas
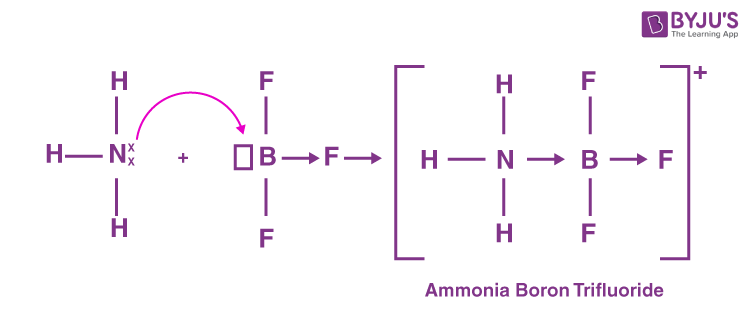
coordination bond
When both the electrons in the shared pair can originate from the same atom