Reduction Reactions of Carbonyl Compounds
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
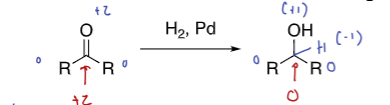
Hydrogen gas with a metal catalyst (H2, Pt or Pd)
ketones and aldehydes to alcohols, but used rarely
not chemoselective
it’s not that it doesn’t happen, it’s just very slow, so it’s unlikely
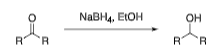
Sodium Borohydride (NaBH4)
ketones and aldehydes to alcohols w/ chemoselectivity
selectively reacts w/ one functional group over others
Lithium aluminum hydride (LiAlH4)
extremely reactive, most carbonyl compounds are reduced to alcohols
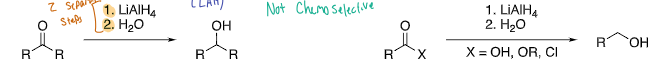
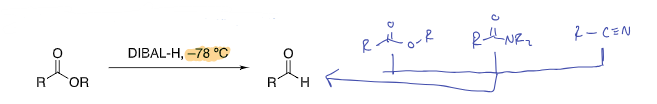
Diisobutylaluminum hydride (DIBAL-H, (i-Bu)2AlH
esters, amides, and nitriles are selectively reduced to aldehydes at low temperature
Aqueous chromic acid (Na2Cr2O7,H2SO4(aq) or CrO3,H2SO4(aq))
Oxidizes primary alcohols to carboxylic acids, and secondary alcohols to ketones
called the Jones Oxidation
Anhydrous Pyridium chlorochromate (PCC)
oxidizes primary alcohols to aldehydes OR secondary alcohols to ketones
Swern Conditions (1. DMSO, (COCl)2; 2. Net3)
oxidizes primary alcohols to aldehydes OR secondary alcohols to ketones
Dess-Martin periodinane (DMP)
oxidizes primary alcohols to aldehydes OR secondary alcohols to ketones
NAD(P)+, H+
biochemical oxidizing agents capable of a wide variety of enzymatic oxidation reactions (*** but don’t use in synthesis)