VSEPR Theory (Molecular Shapes)
0.0(0)
Card Sorting
1/16
Earn XP
Description and Tags
A=the central atom X=an atom bonded to A
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
1
New cards
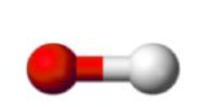
Linear, Linear (1 bonded atom, 0 lone pairs) 180
2
New cards

Linear, Linear (2 bonded atoms, 0 lone pairs) 180
3
New cards
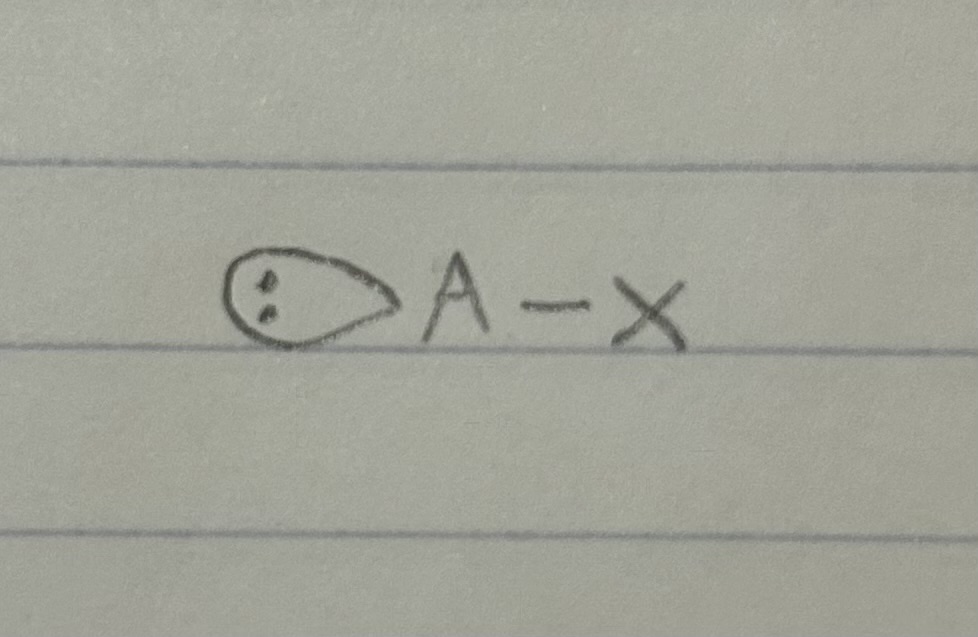
1 bonded atom, 1 lone pair
Linear, Linear (1 bonded atom 1 lone pair) 180
4
New cards
Trigonal planar, trigonal planar 120
5
New cards
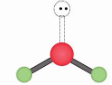
Bent, Trigonal planar 120
6
New cards
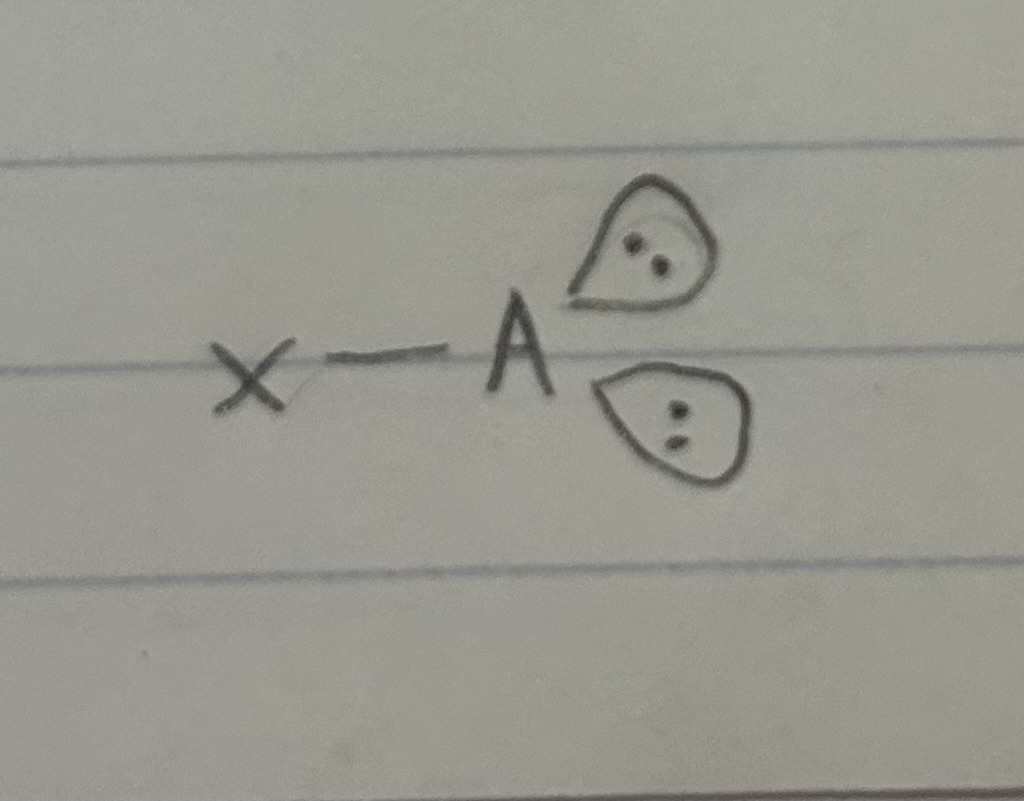
1 bonded atom, 2 lone pairs
Linear, Trigonal planar 120
7
New cards
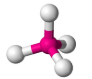
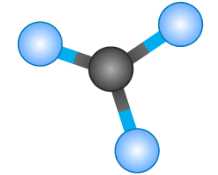
Tetrahedral 109.5
8
New cards
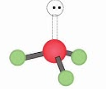
Trigonal pyramid, Tetrahedral 109.5
9
New cards
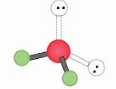
Bent, Tetrahedral 109.5
10
New cards
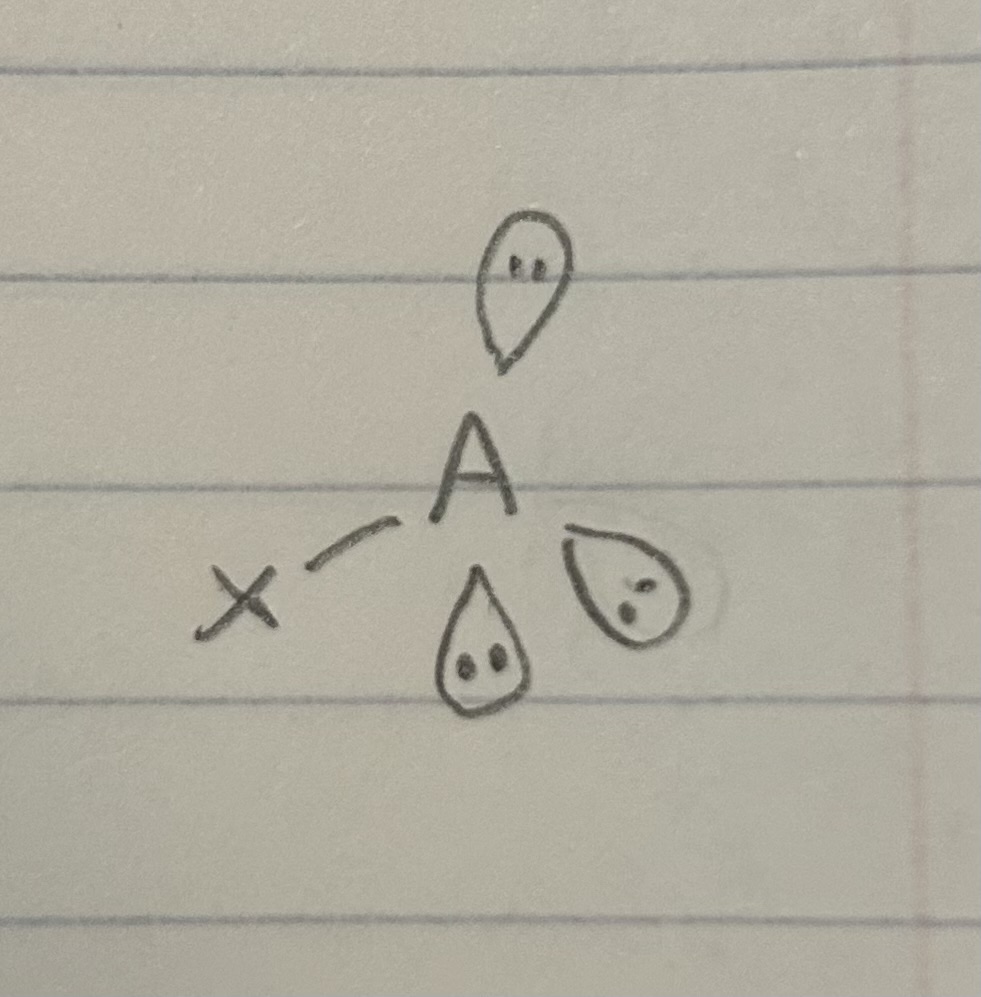
1 bonded atom, 3 lone pairs
Linear, Tetrahedral 109.5
11
New cards
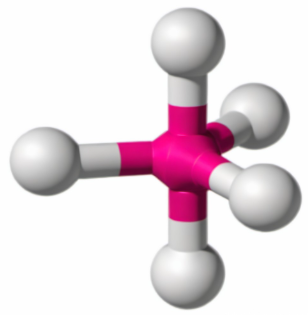
Trigonal bipyramid 90 and 120
12
New cards
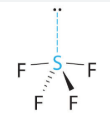
See Saw, Trigonal bipyramid 90 and 120
13
New cards
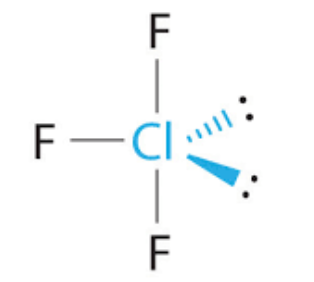
T shape, Trigonal bipyramid 90 and 120
14
New cards
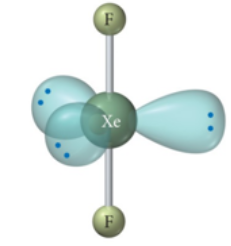
Linear, Trigonal bipyramid 90 and 120
15
New cards
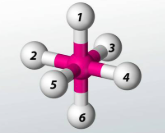
Octahedral 90
16
New cards
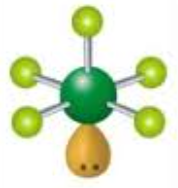
Square pyramid, Octahedral 90
17
New cards
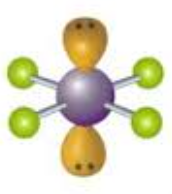
Square planar, Octahedral 90