Lecture 34: Function and regulation of autophagy
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
what is autophagy?
an intracellular self-degradative process found in all eukaryotic cells
what is the role of autophagy in normal cell function?
Clearance of cytoplasm (basal):
removal of aged ribosomes, abnormal protein aggregates, long-lived proteins, invasive viruses, etc.
turnover of excessive/defective/harmful organelles
what is the role of autophagy in stress response?
Nutrient recycling (induced):
in response to new cell development need, starvation, and other stresses it provides
amino acids for protein synthesis
energy source for cell development or survival
what is a cell a balanced state between?
synthesis and degredation (replacement of proteins every 3 months)
new central dogma
DNA → RNA → Protein → folding, trafficking, turnover
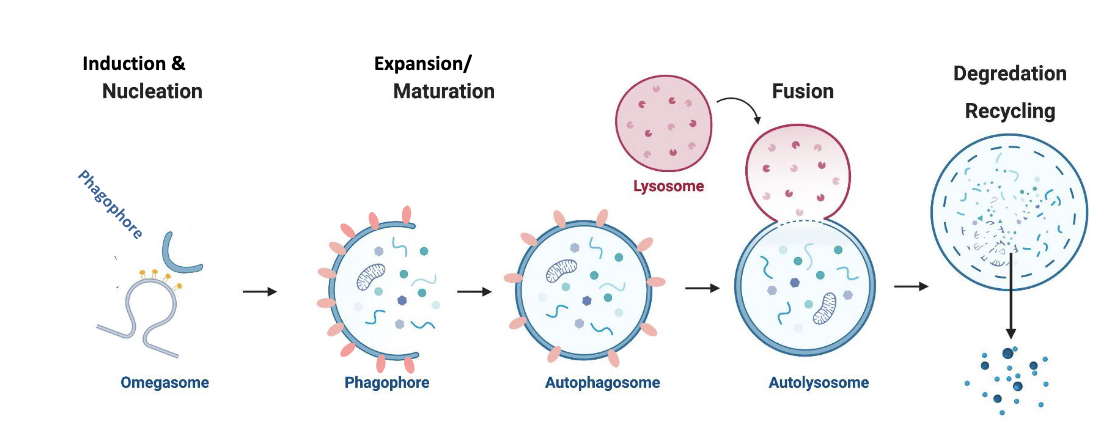
general process of autophagy
omegasome (induction & nucleation) → phagophore → autophagosome (expansion/maturation) → autolysosome (fusion of autophagosome with lysosome) → degradation and recycling
Ohsumi’s genetic screen for yeast apg (atg) mutants
treat cells with a point mutation (EMS), ~38000
first screen for loss of viability in N starvation (wild type survive, ~ 2700 mutants don’t)
second screen for lack of autophagic body accumulation in vacuole/ (99 mutants)
complementation analysis (adding wild-type gene back to mutant to see if it changes)
14 remaining mutants = 14 Apg mutants isolated
what is Atg1?
a kinase that induces autophagy
what happens to Atg1 in conditions where autophagy is favoured?
the protein complex with Atg13 dephosphorylates and fuses to Atg1, and is targeted to the omegasome
what allows phagophores to grow?
lipidated Atg8/ LC3-II
what is used to attach lipids to Atg8?
Atg12-Atg5-Atg16 complex
important note about Atg8/LC3
when on growing phagophores they serve as a cargo ligand that recruits cargoes (ex: defective organelles)
what is autophagy in the mitochondria called?
mitophagy
who supplies membranes for phagophore expansion?
Atg9 vesicles (from ER) and Atg2/Atg18 channels (direct contact with ER)
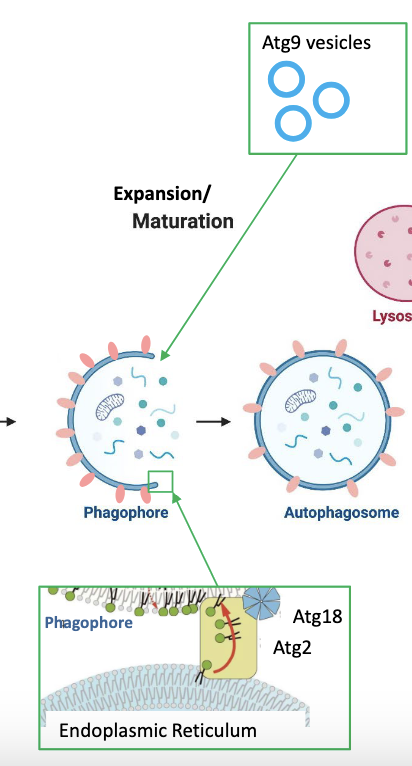
how is Atg18 tethered onto the ER?
It is attached to Rab18, acting as a tethering factor, and when Rab18 goes through GTP hydrolysis it releases the phagophore
what regulates fusion of autophagosomes with lysosomes?
Atg8 / LC3 needs to be released from the autophagosome to allow for fusion:
SNAREs: SNAP29 (and other?)
Rab proteins: Rab7 (?)
Tethering factors: Atg 14
when specifically is autophagy transiently induced and why
immediately following birth to enable newborn to obtain nutrients before it is able to drink from mom and after removal of placenta
what happens to newborns if you knockout Atg5?
there is no lipidation of Apg8, meaning that there is no induced autophagy, leading to no nutrients
how is autophagy used in viral infections?
to clean out pathogens
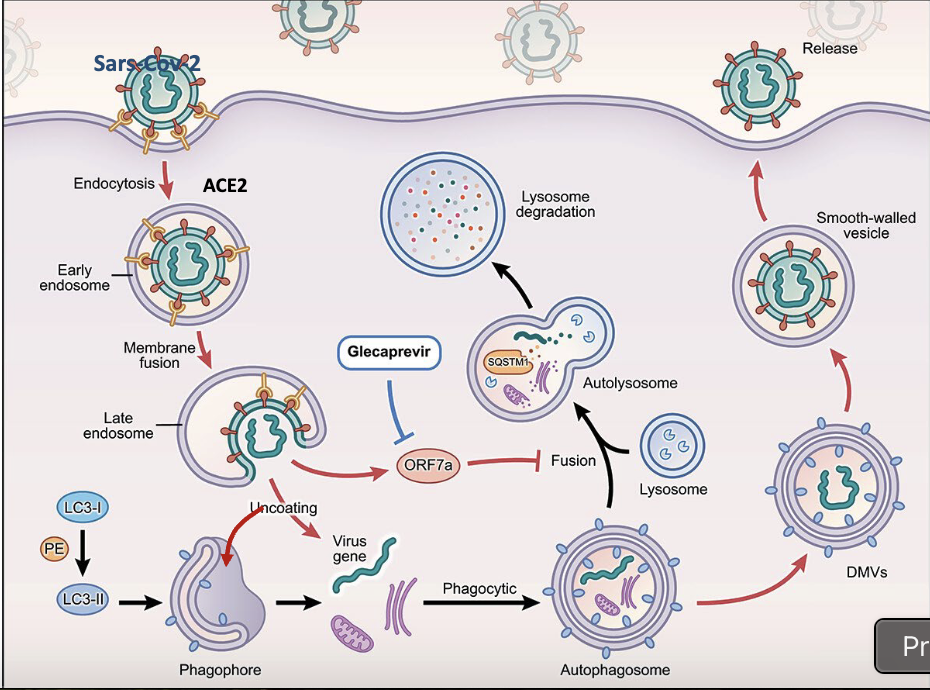
explain process of Sars-Cov-2 in autophagy
spike proteins are recognized by receptors on cell membrane
endocytosis to early endosome
membrane fusion to late endosome, releasing virus gene
virus gene within autophagophore, should go to the lysosome but the protein released (ORF7a) blocks fusion
formation of DMV (double membrane vesicle)
Release of new virus
what inihbits ORF7a?
Glecaprevir, allowing for normal pathway to continue