Chapter 9.2 - Actin and intermediate filaments
1/90
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
91 Terms
intermediate filaments
strong flexible ropelike fibers that provide mechanical support to cells that are subjected to stress
what cells are intermediate filaments found in
animal cells
how are intermediate filaments different from actin filaments and microtubules
chemically heterogenous group of structures that are encoded by approximately 70 genes different genes
how are IFs divided into classes
based on the type of cell in which they are found (as well as biochemical, genetic, and immunologic criteria
t or f - IF assembly does not involve ATP or GTP hydrolysis
true
structure of IFs in the cell
radiate through the cytoplasm of a wide variety of animal cells and are often interconnected to other cytoskeletal filaments by thin, wispy cross bridges
what do many IF cross bridges contain
elongated dimeric protein called plectin
plectin binding sites
has a binding site for an intermediate filament at one end and depending on the isoform a binding site for another intermediate filament, microfilament, or microtubule at the other end
basic building block of IF assembly
thought to be a rodlike tetramer
how do the tetramers of IFs assemble
eight tetramers associate with one another in a lateral arrangement to form a filament that is one unit in length
do IFs have polarity
no, its tetrameric subunits do not have polarity which distinguishes it from IFs from other cytoskeletal elements
IF solubility compared to other cytoskeletal elements
less sensitive to chemical agents than other types of cytoskeletal elements and more difficult to solubilize
what happen when labelled keratin subunits are injected cells
rapidly incorporate into existing IFs, not incorporated at the ends of the filament but into the filaments interior
how are IFs assembly and disassembly controlled
controlled promarily by subunit phosphorylation and dephosphorylation
keratins are what type of cytoskeletal element
IFs
what residue do keratins tend to have a lot of
cysteine
most diverse IF family
keratins
three major components of the nuclear envelope
nuclear pores
nuclear membranes
nuclear lamina
what happens to the nuclear envelope at the end of prohase
disassembles
what is the nuclear lamina composed og
intermediate filaments (lamin) and membrane associated proteins
how are lamina disassembled
phosphorylated which causes de-polymerization and subsequent disassembly of the lamina
epidermolysis bulliosa simplex (EBS) arises from what
mutations in a gene that encodes a keratin polypeptide
desmin related myopathy
mutations in the gene that encodes desmin
what does desmin do
integrates the various components of a muscle cell
what does desmin related myopathy lead to
skeletal muscle weakness, cardiac arrythmias, and eventual congestive heart failure
functions of microfilaments (actin)
motility
shape
structural support
muscle contraction
most abundant protein in cells
actin
g actin
globular
f actin
filaments
what happens to actin monomers in the prescnce of ATP
polymerize into a flexible helical framework
minus end of actin
the end of the filament with an exposed binding cleft, binds plus end in a filament
plus end of actin
the other end is where the minus end of G actin binds
pointed end
minus end of actin
barbed end
plus end of actin
how is the ATP binding cleft oriented in actin
in the same direction in all actin subunits (monomers) in the filament
in vitro polymerization of lus and minus ends of actin
have different polymerization rates
in vivo polymerization rates of the plus and minus ends of actin
polymerization only ocurs in the plus end
why does tee plus end only polymerize in vivo for actin
minus end MAY be anchored
what dissociates in actin
only ADP-actin dissociates
how is actin polymerization and organization regulated
actin binding proteins
what dies actin bind to a lot in eukaryotic cells
many many acessory proteins
what are myosins (type of proteins)
actin based motor porteins
what do all mysoins have in common
have a characterituc head or motor domain (ATP-ase activity)
how are myosins goruped
grouped into conventional and unconventional myosins
what direction to myosins move
to positive end except myosin VI
conventional myosins
type II
what are type II myosins composed of
6 polypeptide chains
ine pair of heavy chains
two pairs of light chains
symmetry of type II mysoins
highly asymmetric protein
mysoin II consists of
a pair of globular heads that contain the catalytic site of the molecule
a pair of necks, each consisting of a single, uninterrupted α helix and two associated light chains
a single, long, rod-shaped tail formed by the intertwining of long a-helical sections of the two heavy chains
in what way does myosin II assemble
into fibers with the ends of the tails pointing toward the center and the globular heads pointing away
skeletal muscle is used for
voluntary movement
muscle
bundles fo parallel muscle fibers (cells) joined by tendons to the bones that the muscle must move
fibers
each fiber is a multinucleate cell formed during embryogenesis and specialized for contraction
myofibril
thinner cylindrical strands that make up a muscle fiber and consist of repeating units of sacromeres
sacromeres
the contractile unit of myofibrils each of which hs a very specific organization
organization of sacromeres
each sacromere extends from one Z line to the next Z line
thin filament
actin
thick filament
mysoin
actin orientation
plus ends anchored at Z lines
capZ
caps actin at the plus end
tropomoulin
caps the minus end and regulates the length of actin filaments
nebulin
repeating actin binding motifs that binds actin filament to Z line
myomesin
bundles of the myosin filaments
titin
extends through the myosin filaments (thick) and attaches to the Z line - helps prevent tearing of muscle
sliding filament model of muscle contraction
During contraction, the myosin molecules pull the surrounding thin filaments (actin), forcing them to slide toward the center of the sarcomere
Individual myosins work asynchronously, so that only a fraction are active at any given instant
The “neck” acts as a lever, amplifying the conformational change caused by ATP hydrolysis
contrctile cycle (what is it)
Conformational changes (mechanical) in the myosin head couple ATP hydrolysis (chemical) to movement
contractile cycle steps
ATP binds to the cleft in the myosin head, releasing myosin from actin
ATP hydrolysis to ADP+Pi causes weak binding to actin
Pi release causes tighter binding and the power stroke that moves the thin filament toward the center of the sarcomere
ADP is released, freeing the ATP binding cleft 1) ATP binds to the cleft in the myosin head, releasing myosin
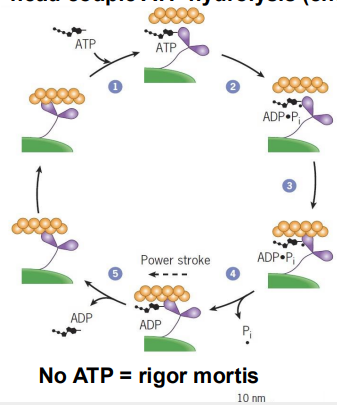
what do calcium ions trigger and how
contraction via troponin and tropomyosin
tropomyosin
masks the myosin binding sites on the actin filament
troponin complex has how many subunits
3
troponin complex
binds to tropomyosin
role of tropomyosin and troponin
both have regulatory roles in contraction
calcium binding to troponin ____
relieves th etropomyosin blockages of the interaction between actin and myosin head
steps of contraction due to calcium
Motor neuron excitation signal
Signal transduction pathway leads to Ca2+ release from the SR
Ca2+ binds to troponin (TnC subunit), causing conformation shift
Troponin conformation shift moves tropomyosin out of place
Myosin binding site on actin is exposed
When excitation signaling ceases, Ca2+ are pumped back into SR, muscle relaxes
Familial hypertrophic cardiomyopathy:
Genetically dominant inherited mutation in myosin (~2 per 1000 people)
Familial hypertrophic cardiomyopath can cause
Over 40 different point mutations can lead to:
Heart enlargement
Abnormally small coronary vessels
Cardiac arrhythmias
cofilin
binds ADP actin and severs filaments promoting depolymerization
profilin
functions as an adenine nucleotide exchange factor
Binds to ADP actin (at the plus end), changing the conformation and allowing binding of ATP
Binding results in dissociation of profilin
ATP actin then either joins a growing filament at the plus end OR is bound by:
thymosin
sequesters G- actin preventing polymerization
Displacement of thymosin allows binding of G-actin to the plus end
whta deos cpping do in F actin
stabilizes it
what does capping the plus end do in f actin
prevents further growth
what does capping the minus end do in f actin
prevents to loss of subunits
in muscle, which en of f actin is capped
both, prevents the loss or gain of subunits this way
what can actin be lined to
other actin filaments or indirectly to the cell membrane
cell cortex
network of actin filaments and accessory proteins that underlies the plasma membrane in most eukaryotic cells
Arp2/3 complex
actin related proteins, nucleates new branches off the sides of existing filaments
what are Arps activated by
WASPs (Wiskott-Aldrich syndrome protein)
to what end is g actin added
plus end
how can filaments move the cell membrane forwards
1 and 2) Extracellular signal recognized and signal transduction cascade initiated
3 and 4) WASP activates Arp complex and Arp nucleate new actin filaments
5) result is branch formation at plus end
6) Membrane is pushed forward
7) Caps terminate elongation
8) Oldest part of filament at the minus end
9) F-actin servered and depolymerized at minus end
10) Profilin exchanges ADP to ATP
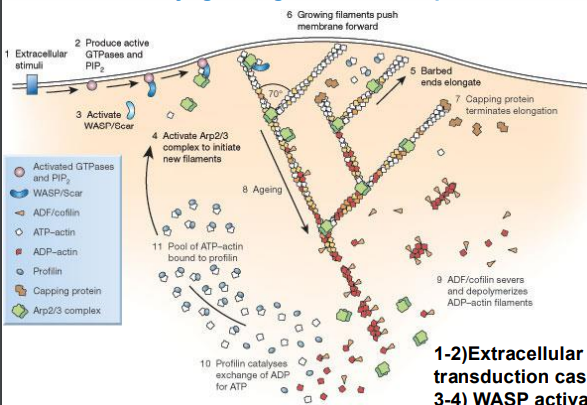
what regulates the actin skeleton
Rho family of small GTPases
where is Rho bound and what does that do
Rho family GTPases often are bound to a guanine nucleotide dissociation inhibitor (GDI) in the cytosol
The GDI prevents Rho from interacting with it’s GEF at the plasma membrane