Lecture 11 - Principles of vaccines
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
define immunization
the act of making someone immune to a particular disease
define vaccination
the deliberate induction of an adaptive immune response by injecting a vaccine (dead, attenuated, non pathogenic) form of a pathogen
types of immunity
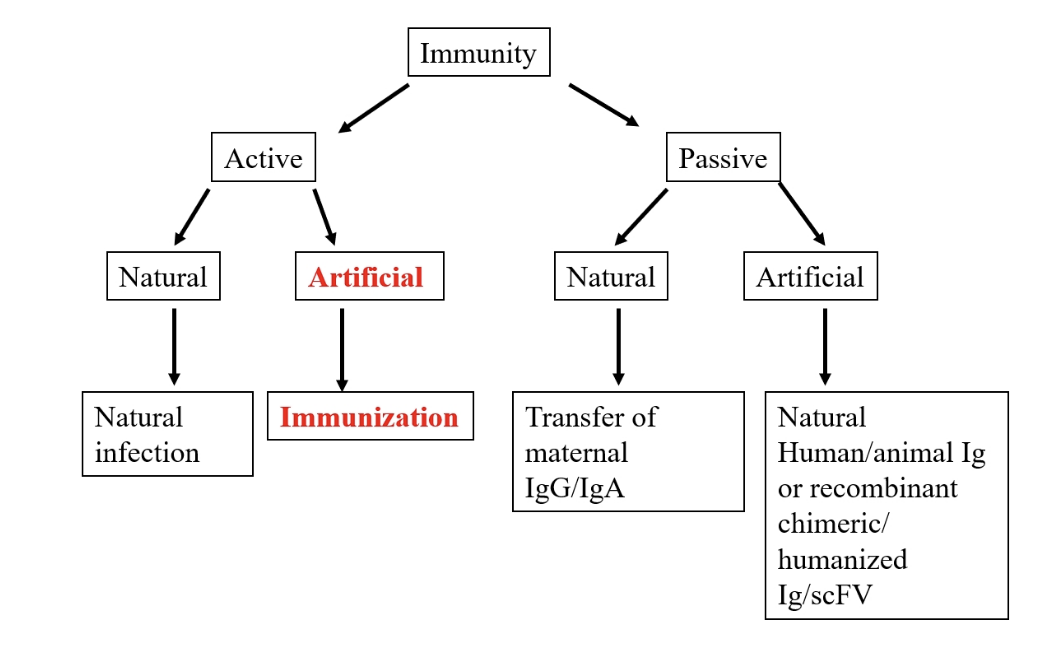
passive immunity
passive transfer: protection by transfer of specific, high titre, Ab from immune donor to a non immune recipient
eg: isolating horse Abs for Hep B
adoptive transfer: immune cells from an immunised individual
immediate protection
only offers transient immunity
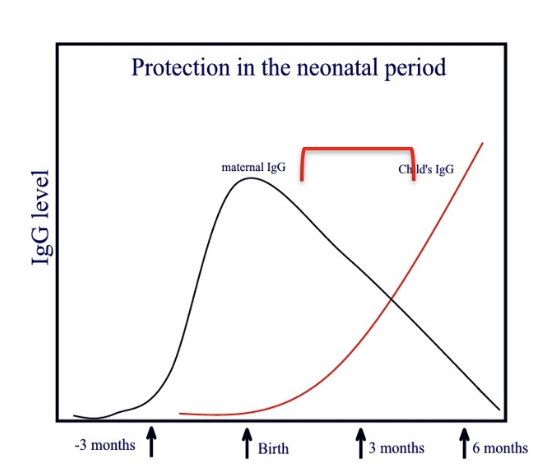
Naturally aquired passive immunity
required for neonatal protection
IgG transferred from mother to foetus
IgG trough 3-6moths due to decline in maternal IgG
Maternal IgA transferred through colostral transfer
artificial passive immunity
pooled specific immunoglobin
animal sera (anti-toxins, anti venoms)
usually isolated from immunized large animals
eg horses used to neutralise toxins and venoms
stop them binding to target receptors on cell surfaces
Passive immunisation: Ebola
Ebola virus infection causes a deadly heamorrhagic disease
(50% - 90% death rate)
two licensed vaccines: monoclonal antibody therapies that target EBOV GP
EBOV encoded glycoprotein (GP): affects ability of virus to bind to and infect cells.
EBOV mainly infects endothelial cells, mononuclear phagocytes and hepatocytes
via C-type lectins, DC-SIGN and integrins
secreted glycoprotein (sGP): essential role in the pathogenesis of Ebola Virus Disease (EVD).
exapmles of Ebola virus passive therapies
PALM Trial: 681 patients November 2018 – August 2019
Patients treated with
REGN–EB3 (Inmazeb) – cocktail of 3 McAb which bind to EBOV GP (31% DR)
Ebanga (Ansuvimab – previously m114) – isolated from immortalised B cells from a survivor of 1995 outbreak in Congo, Binds to EBOV GP (35% DR)
Zmapp – cocktail 3 McAb used in previous outbreaks (50% DR)
Remdesivar - antiviral drug (53% DR)
Trial stopped and licence granted to REGN-EB3 and Ebanga (Oct and Dec 2020)
coronavirus and mAb Therapies
Spike 1: receptor binding domain
Many potential Monoclonal Antibody Therapies for SARS-COV but
none licensed to date: immuno-evasion by new variants
Why vaccinate?
gain immunity after recovery: memory B cells
less susceptible to same pathogen
less severe sypmtoms
passive Ab therapy is best transient
Chinese variolation: scabs from small pox survivors
UK Edward Jenner: milkmaid and cowpox
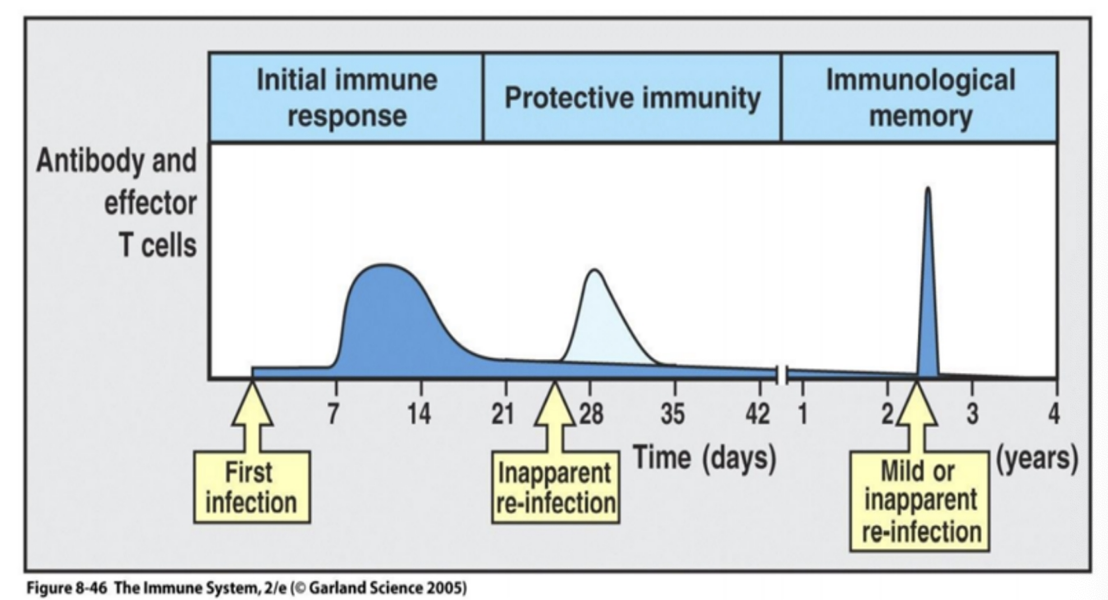
Immunological memory
immune system can respond more rapidly and effectively to pathogens that have been encountered previously
Reflects the pre-existence of a clonally expanded population of antigen- specific T and/or B lymphocytes.
memory B cells generate high affinity IgG
The goal of vaccines: artificially induce a long lived immunological memory in a host to protect against subsequent re- infection.
Can last the life-span of the host – smallpox, yellow fever, polio, measles etc
B Cells
The secondary response:
larger frequency of Ag specific cells (memory cells)
long lived: present after the primary response
proliferate more rapidly
produce bore Ab
Produce Ab with specialised effector function: IgG + IgA
Ab have higher affinity
better than naïve B cells
During initial expansion of Ag specific B cell clones, some progeny cells do not develop into plasma cells
they revert to small lymphocytes that maintain same Ag-specific BCR on their surface (memory B cells).
Somatic hypermutation in B Cells
a secondary immune response produces Ab with higher affinity
SHM caused alterations in variable regions of light + heavy chain of memory cell Ab
random process
T Cells
Memory T cells are long lived, have increased frequency and proliferate more rapidly than naïve T cells.
Naïve T cells express the tyrosine phosphatase CD45RA which does not associate with the TCR
Memory T cells express CD45RO which associates with both the TCR and co-receptor (CD4).
This complex tranduces signals more effectively than the receptor on naïve T cells.
Types of T cells
Effector memory T cells
upon Ag re-stimulation, rapidly mature into effector cells
move into tissues
lose CCR7 expression
Release lots of effector associated cytokines
e.g. IFN-g and IL-4
Central memory T cells
mature into effector cells slower
maintenance of CCR7: stay in lymph node for longer
Take longer to secrete effector associated cytokines
Central Memory T cells CD45RO+ve and CCR7 +ve ,
Effector Memory T cells CD45RO +ve and CCR7 -ve
If the aim of a vaccine is to induce memory cells – what must it do?
Be captured and processed by APC for MHC presentation to activate T cells via TCR (generates Signal 1)
class- switched antibody responses require T cell help
Activate innate cells (including APC) via Pattern Recognition Receptors PRR.
Activated APC will express co-stimulatory molecules and so deliver Signal 2 (B7 on APC interacting with CD28 on T cell) and Signal 3 (cytokines) to activate T cells
Induce high levels of T and B cell primary activation, with a high efficiency of generating both T and B memory cells.
Contain several epitopes that are recognized by several TCRs and BCRs in order to activate multiple T/B clones (to counter any antigenic variation).
Provide a constant and long lasting source of Ag in lymphoid tissue.
To induce a protective response(s) to pathogen without causing disease.
Requirements of an effective vaccine
Safe (minimal side effects)
high level of protection
long-lasting protection
right type of response (local, systemic, B or T cells or both)
Efficacy of vaccine depends on many people being vaccinated (Herd Immunity), so must be:
Low cost
Stable (in high temperatures)- cold chain requirements
Easy to administer
Minimal side-effects
Common Vaccination Approaches
1. Live attenuated vaccines
2. Killed vaccines
3. Sub-unit vaccines
4. Conjugate vaccines
5. Recombinant vaccines
Live Vaccines
Measles, mumps, rubella, oral polio (Sabin), BCG, Yellow fever.
Attenuated (e.g. oral polio (Sabin)) - cold attenuated, host range mutants, long term culture, adapts to culture environment, so dies in humans before xausimg disease
The measles virus used as a vaccine today was isolated from a child with measles in 1954
In 1988 wild polio virus (WPV) was found in 125 countries with some 350 000 children being paralysed.
Fallen to 12 cases (2023) in 2 countries
Problems with live attenuated vaccines
If a population is seriously under-immunised, there will be susceptible children
If the vaccine-virus circulates for a prolonged period it can mutate and over the course of 12-18 months reacquire neurovirulence.
These viruses are called circulating vaccine-derived polioviruses (cVDPV)
800 cases in 10 years
If a population is fully immunized against polio, it will be protected against the spread of both wild and vaccine strains of poliovirus.
pros of live vaccines
Single dose effective
May be given by natural route (oral)
May induce local (gut) and systemic (blood) immunity
May induce right type of response (IgA)
cons of live vaccines
Reversion/alteration to virulence (Sapolio - cVDPV)
Possibility of contamination
e.g. Hep B contamination of Yellow Fever Vaccines
Susceptible to inactivation - heat
Can cause disease in immunocompromised host
Killed vaccines
Examples of killed vaccines: include (Salk polio vaccine), pertussis (whooping cough), typhoid, cholera.
must survive killing
May have side effects
A common way to kill organisms is with chemicals such as formaldehyde
Advantages of killed vaccines:
stable in storage
will not cause disease through residual virulence and cannot revert
(Purified) subunit vaccines
made from biochemically purified components of pathogens
components must elicit protective immune responses
Examples: HiB (Haemophilus influenza B which causes meningitis, pneumonia etc)
The vaccine is made from purified capsular polysaccharides.
Influenza vaccine = purified haemagglutinin (H) + neuraminidase (N) antigens of the particular strain that is prevalent
(Recombinant) subunit vaccines
To avoid the problems involved in bulk culture of pathogens recombinant vaccines have been introduced
by inserting DNA of antigen into bacteria to express
Hepatitis B was the first recombinant vaccine licensed for human use
inserted antigen DNA in yeast
Ab to Hepatitis B surface antigen (HBsAg.) are protective in natural infections
Respiratory Syncytial Virus – subunit vaccine introduced by NHS September 2024 – Pregnant Women and adults 75-79
Contains RSV Subgroups A and B stabilised recombinant F antigen.
Conjugate vaccines
Young children don’t make an immune response against carbohydrates
B cell binds to an internalised bacterial polysaccharide, can’t present for the T-cell
Haemophilus influenzae type b capsular polysaccharide (carbohydrate) conjugated to tetanus toxoid
Converts TI-2 polysaccharide antigen to a TD form
peptide are presented to T-cell
activated Bcell produces Ab against polysaccharide Ag
Young children can respond
Conjugation of a T Independant (non-protein) component to a TDepentant (protein) component has to be performed biochemically rather than using recombinant DNA technology
Other considerations when chosing a vaccine
Different pathogens require differential immune responses: Adjuvants
Adjuvants
Highly purified antigens alone often fail to induce strong immune responses.
Early studies showed impure antigens or antigens mixed with whole bacteria triggered better immunity.
This is because antigen-presenting cells (APCs) need activation by PAMPs (pathogen-associated molecular patterns) or DAMPs (damage-associated molecular patterns) binding to pattern recognition receptors (PRRs).
Adjuvants enhance immune responses by mimicking these natural signals, but stronger adjuvants sometimes cause more tissue damage.
For non-live vaccines, safe and effective adjuvants are crucial.
Aluminum salts were among the first adjuvants used with diphtheria and tetanus vaccines, and remain in use today (e.g., HepA, HepB)
Aluminum activates the Nalp3 inflammasome in APCs, aiding immune activation.
Herd Immunity
Immunization needs to be sustained at a population level
Disease declines in a population if the majority of the population are immune (herd immunity)
Estimated thresholds of population immunity for vaccine preventable diseases:
Mumps 75% - 86%
Smallpox 83%-85%
Pertussis 92% - 94%
Covid-19 Unknown
Requires continuous immunization to avoid a pool of susceptible hosts
Herd Immunity and Whooping Cough
Immunization levels fell to 31% in 1978 due to some doctors suggesting that he vaccine could cause brain damage
Between 1977 & 1982 >110,000 cases
26 children died; similar numbers suffered brain damage
Herd Immunity and MMR
MMR vaccination introduced in 1988
Bad publicity linking MMR vaccination to autism and bowel disease (1998) led to a decrease in uptake of MMR and mumps outbreaks
Since 2004 the majority of confirmed cases = outbreaks in Universities.
Current Worldwide Vaccination Projects
Tuberculosis Vaccine
Malaria Vaccienes
Tuberculosis Vaccine
The tuberculosis vaccine, BCG, was first used in 1921
most widely administered vaccine globally.
BCG contains = live attenuated strain of Mycobacterium bovis
Children: prevents TB meningitis
Adults: little efficacy against pulmonary disease
Tuberculosis is caused by Mycobacterium tuberculosis.
2022 1.3 million people died
Tuberculosis subunit vaccine showed 50% protection against development of pulmonary disease in phase II trial (2019)
large phase III trial launched in March 2024 across South Africa and six other countries
Malaria Vaccienes
Malaria causes 250 million acute clinical cases per year.
0.6 million deaths annually
The malaria parasite has a complex life cycle in both humans and mosquitoes
plasmodium: bite> liver> blood
each life cycle stage has distinct Ag
Many Phase I and II malaria vaccine trials have shown limited clinical success.
Recently licensed vaccines:
Mosquirix 2021 = circumsporozoite antigen + Hepatitis B surface antigen (36% reduction clinical malaria)
Matrix-M 2023 = subunit sporozoite + Hepatitis B surface antigen with Matrix-M adjuvant (77% reduction symtoms)
New sporozoite vaccine study (Olivia AC et al, 2024):
Used genetically modified sporozoite that halts development in the liver 6 days post-infection.
GA2 (late-arrested) group showed significantly higher protection than GA1 (early-arrested) group (12.5%).
Potential issues with administration (delivery via mosquito bites)