Module 11: Programmed Cell Death
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
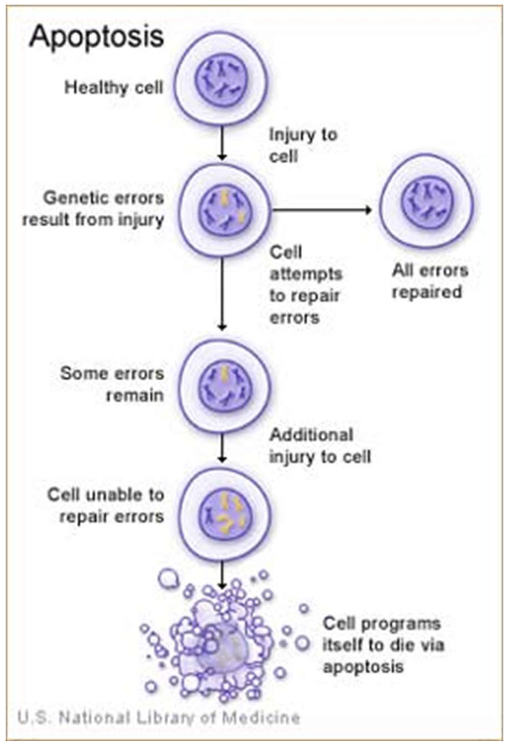
Why does apoptosis occur?
Maintains balance between cell growth and death
50–70 billion cells die daily via apoptosis
Prevents diseases like cancer (too little cell loss)
Dr. Horvitz won Nobel Prize for apoptosis research in C. elegans
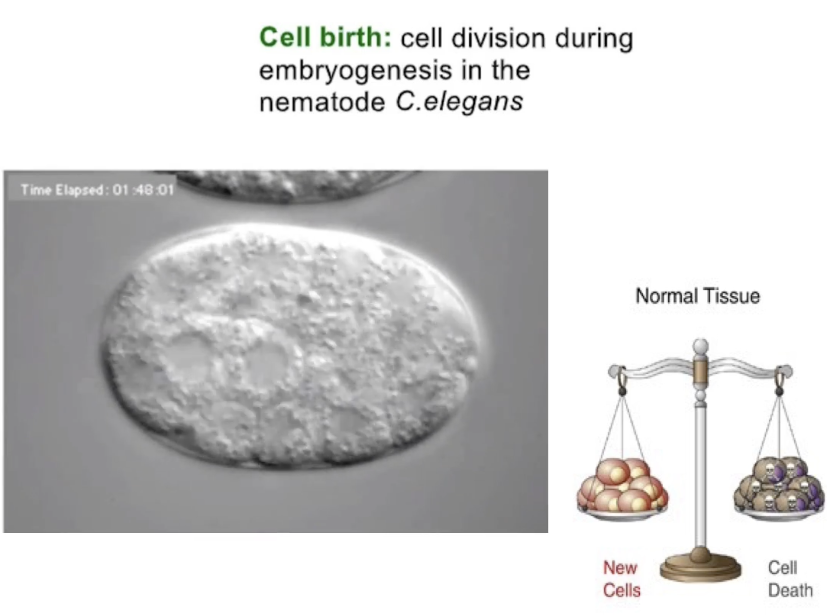
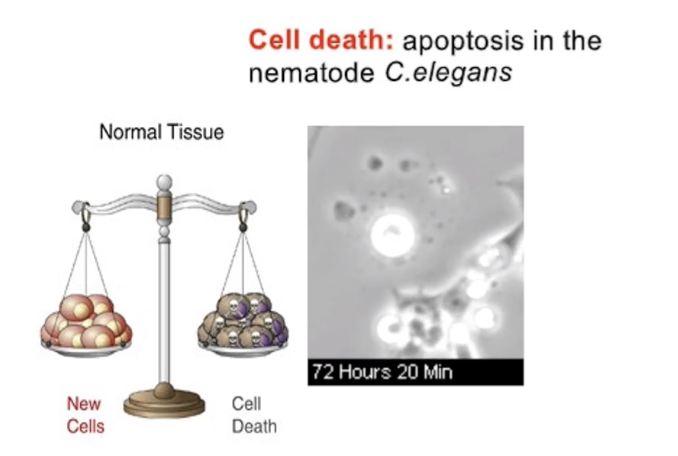
What regulates apoptosis in cells?
A network of signaling proteins
Just like mitosis, apoptosis is highly regulated
Triggered by internal/external signals
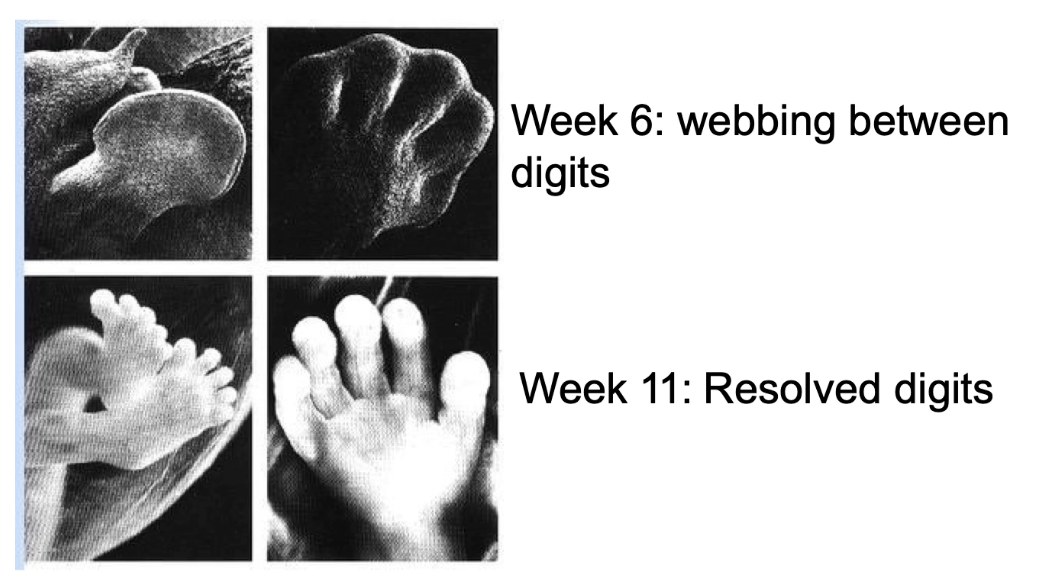
How is apoptosis important during development?
Shapes body structures by removing excess cells
Example: webbing between fingers/toes (week 6) removed by apoptosis (week 11)
Without apoptosis → webbing remains
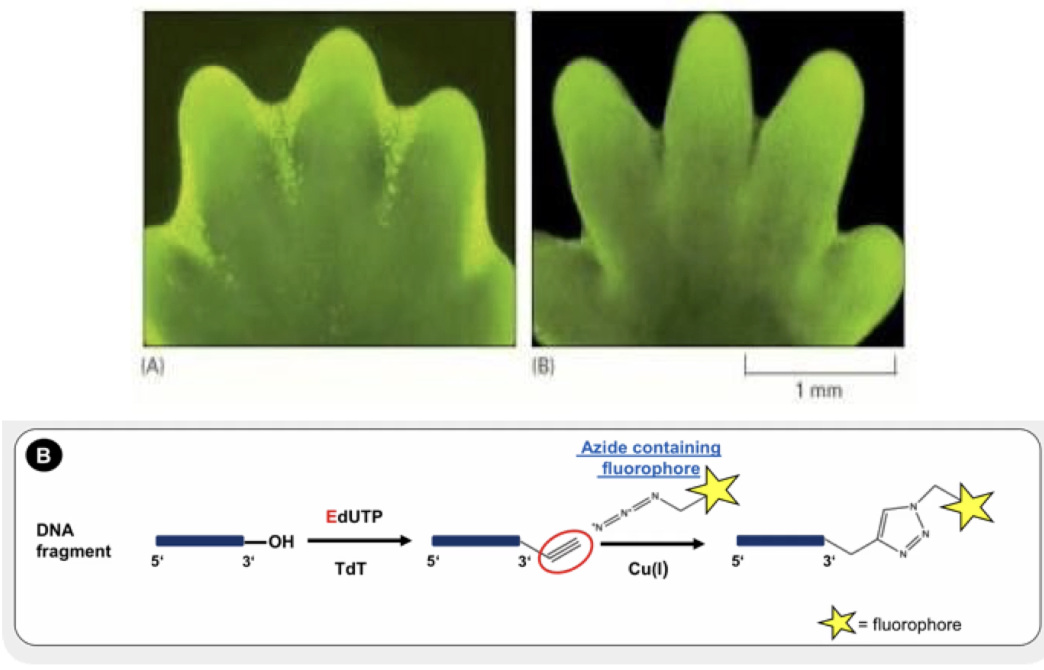
What does the TUNEL assay detect?
Cells undergoing apoptosis
Stains DNA breaks with fluorescent dUTP by terminal deoxynucleotidyl transferase
Bright green dots = apoptotic cells
Shows cell death between developing mouse digits
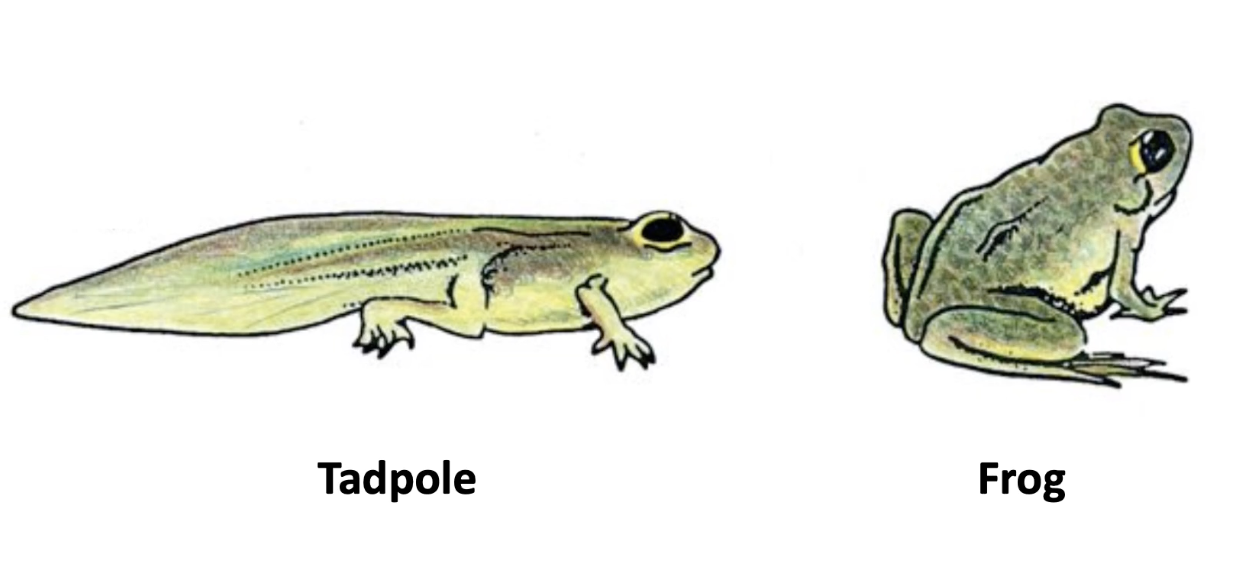
How does apoptosis affect tadpole development?
Causes tail loss during metamorphosis
Triggered by thyroid hormone
Similar tail loss happens in human embryo
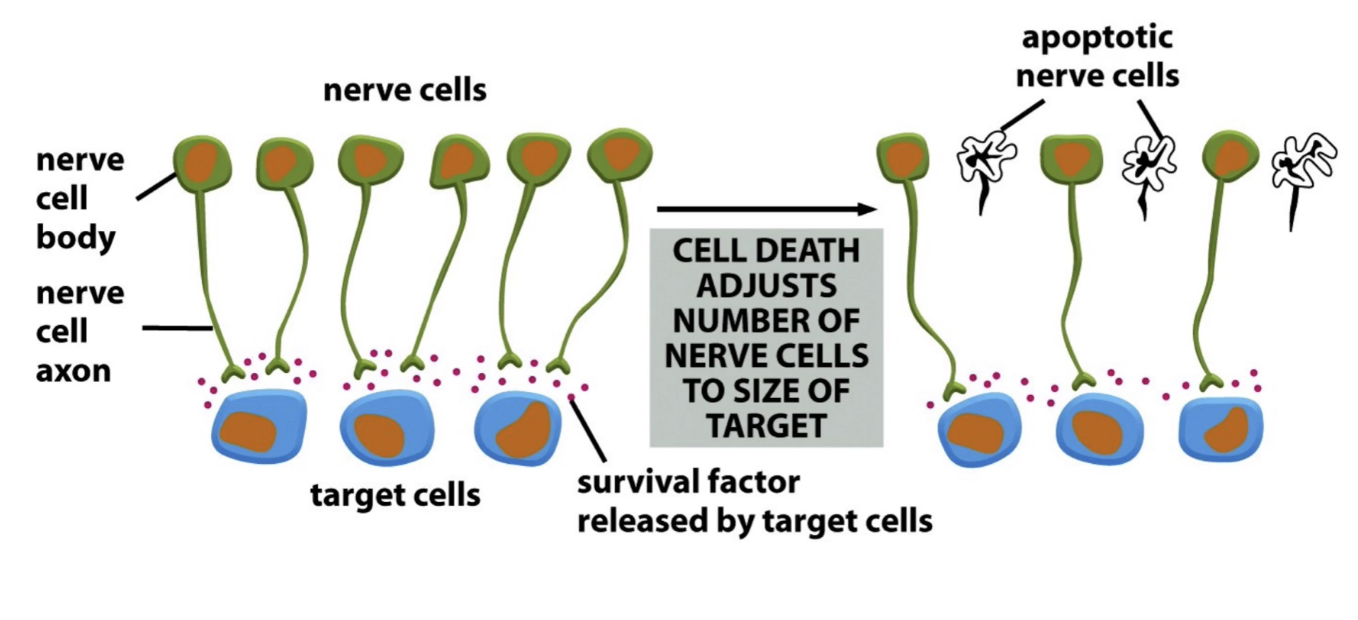
Why is apoptosis important in brain development?
Removes neurons that don’t make proper connections
Up to 50% of neurons die
Ensures correct matching of nerve and target cells
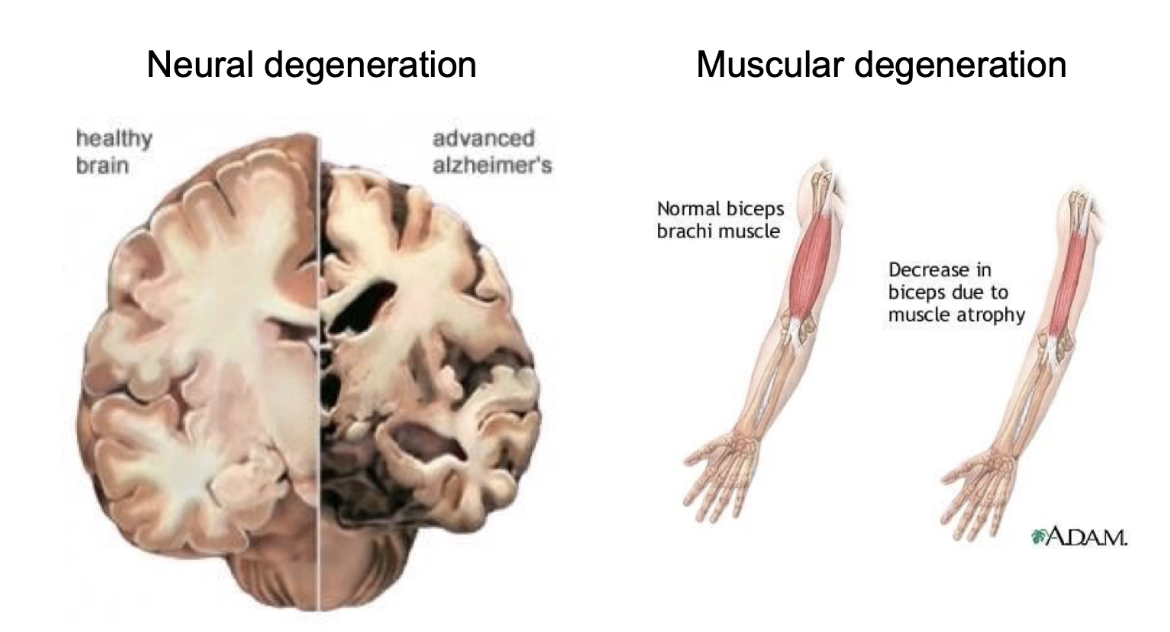
How can apoptosis be harmful?
Excessive apoptosis leads to disease
Alzheimer’s: hippocampus neuron death and certain parts of cerebral cortex
Huntington’s: striatum neuron death
Parkinson’s: dopamine neuron loss in substantia nigra
Duchenne muscular dystrophy: muscle degeneration from cell death
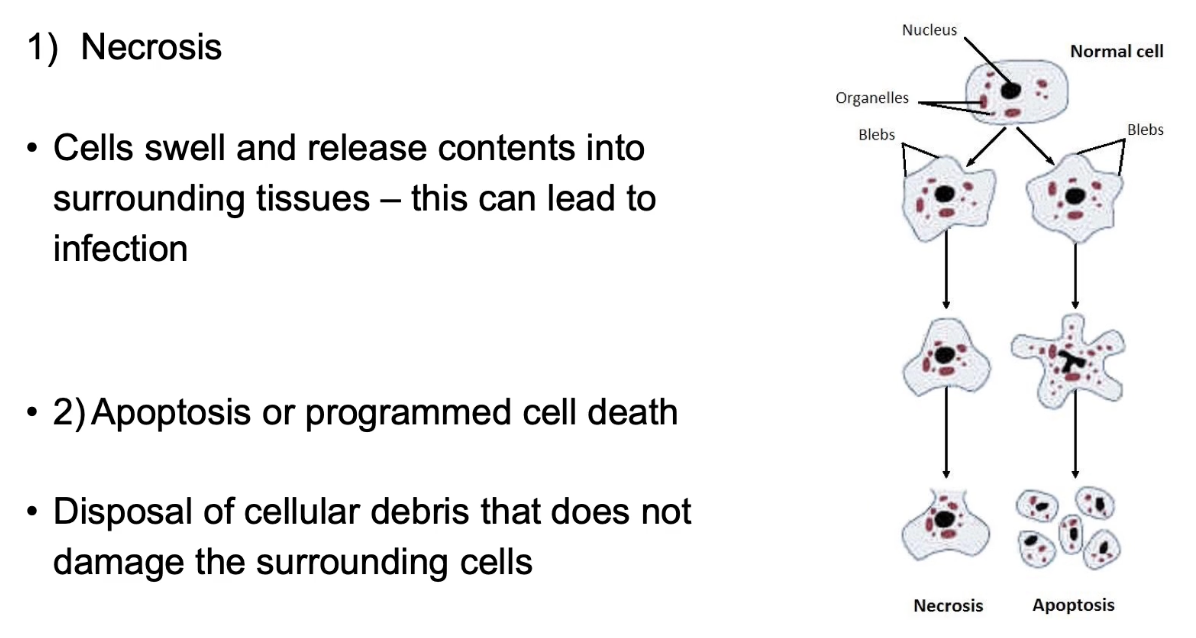
What are the two types of cell death?
Necrosis and apoptosis
Necrosis = unregulated, causes inflammation
Apoptosis = controlled, clean, and safe cell removal
Apoptosis prevents damage to nearby cells
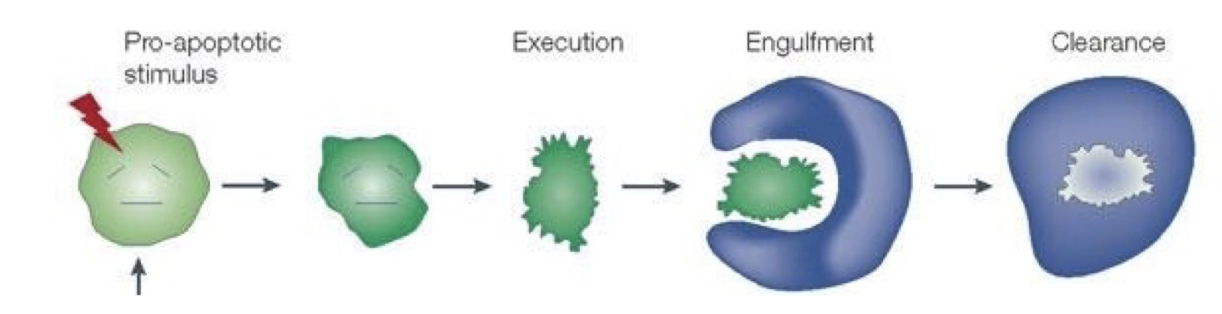
What are the three stages of apoptosis according to Dr. Horvitz?
Cell execution
Engulfment
Clearance
Dr. Horvitz studied cell execution in C. elegans
Apoptosis is an active, stepwise process
What changes occur in a HeLa cell during apoptosis?
Cell shrinks
Membrane blebbing (protrusions or bulges occur)
Mitochondrial permeability changes
Nucleus and DNA degrade
Ends in formation of small, recyclable vesicles

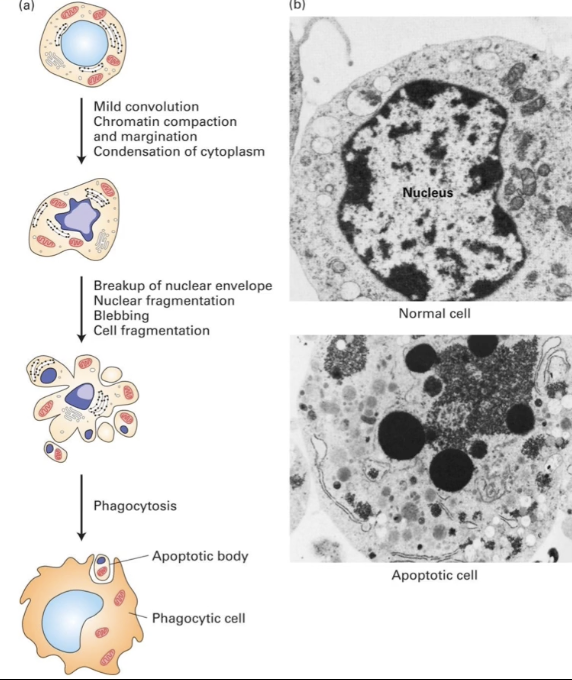
What structural changes occur in an apoptotic cell?
Nucleus:
Chromatin condenses
Nuclear envelope breaks down
DNA fragmented
Proteins degraded
Cytoplasm:
Condenses as components aggregate
Mitochondria:
Membrane becomes permeable
Proteins released into cytosol
Cell membrane:
Changes shape → forms blebs (protrusions)
Cell fragments into vesicles
Outcome:
Vesicles (apoptotic bodies) phagocytized
Cell contents recycled
SEM image comparison:
Normal cell: intact membranes, visible chromatin
Apoptotic cell: condensed DNA and far from nuclear membrane, altered membrane shape
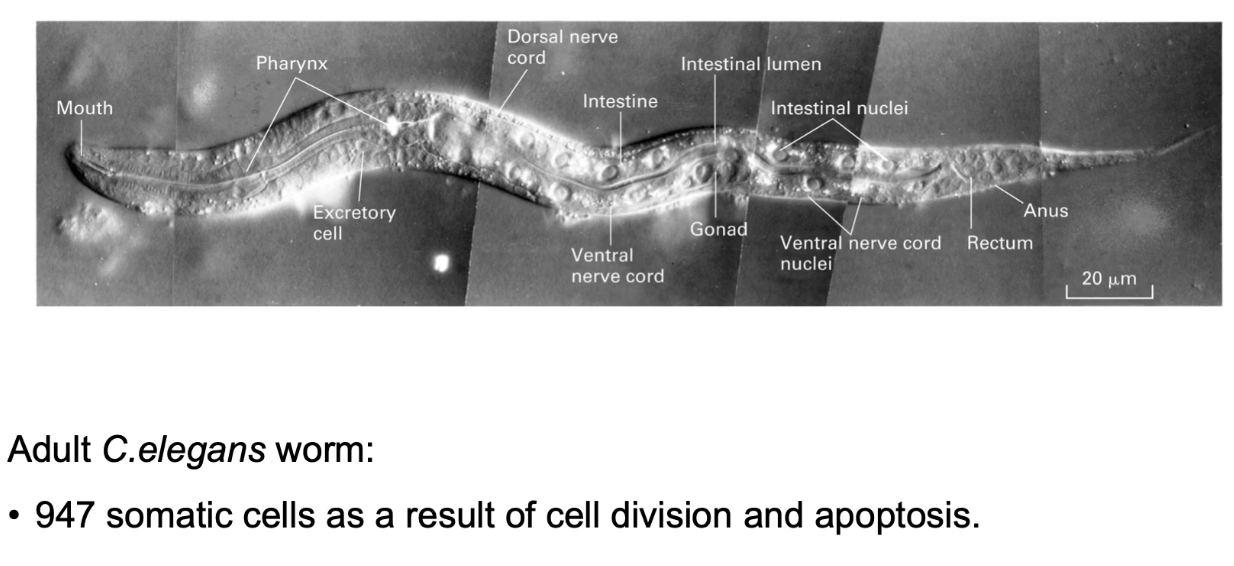
Why is C. elegans a good model for apoptosis studies?
947 somatic cells traced from 1 zygote
131 cells consistently undergo apoptosis
Predictable, well-mapped development
Easy to study effects of gene mutations
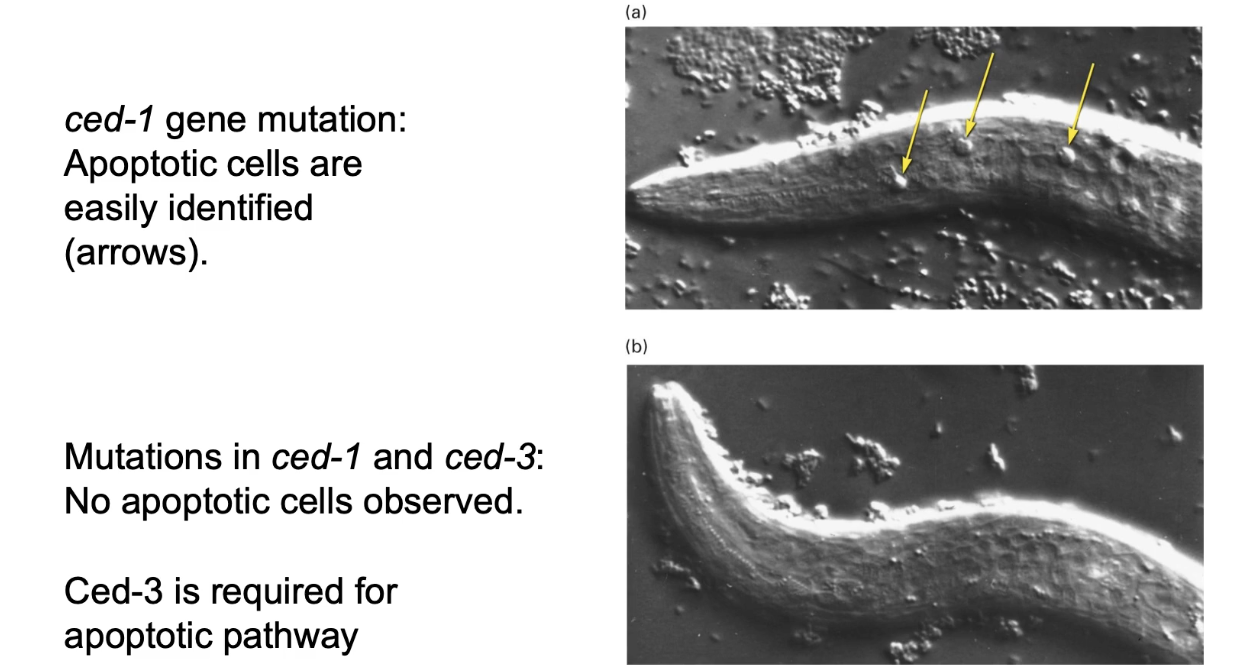
What is the significance of the ced-1 mutation in C. elegans?
ced-1 mutation: cells die but aren’t engulfed by phagocytosis
ced-1 + ced-3 mutation: cells don’t undergo apoptosis
Screen used ced-1 mutants to identify essential apoptosis genes: ced-3, ced-4, ced-9, egl-1
Mammalian homologs exist for all four
Two homologs linked to human tumor formation due to failure in apoptosis
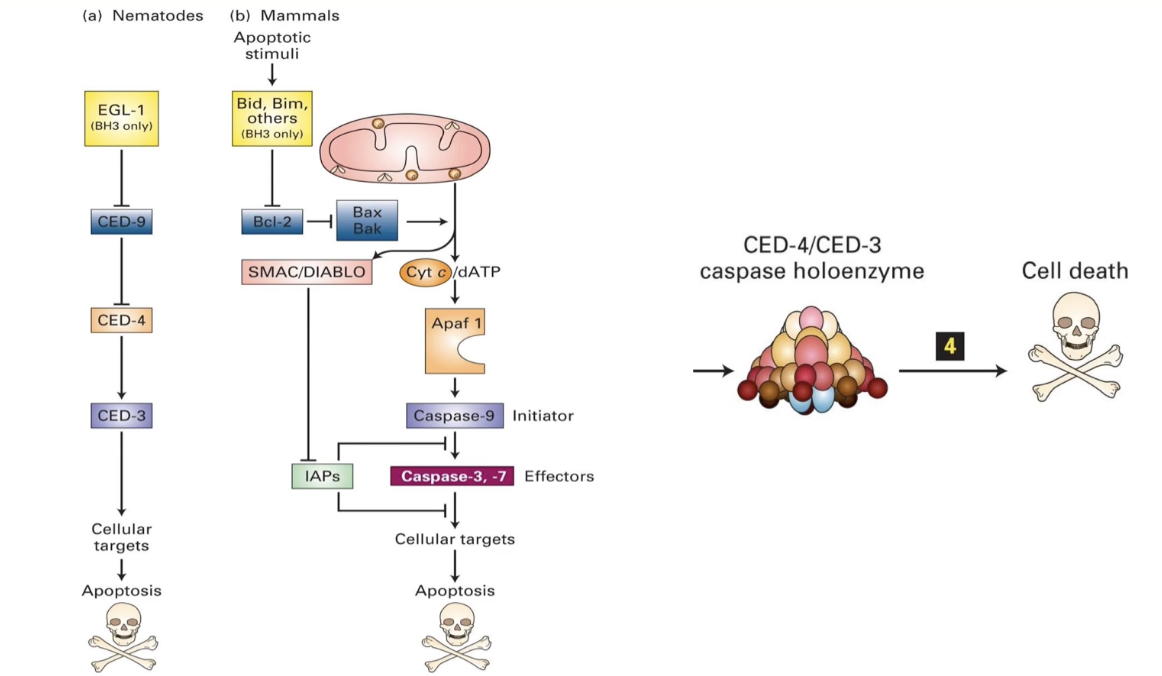
What are the key genes and protein interactions in the C. elegans and mammalian apoptotic pathways?
In C. elegans
ced-3 & ced-4: essential for apoptosis
Form the caspase holoenzyme (a protease)
ced-9: inhibits apoptosis by blocking caspase activation
Loss-of-function → all cells die
egl-1: initiates apoptosis by inhibiting ced-9
In Mammals
EGL-1 homologs: Bid and Bim (BH3 family)
CED-9 homolog: Bcl-2 on mitochondrial membrane
Inhibits apoptosis; controls Bak/Bax (pro-apoptotic proteins)
CED-4 homolog: Apaf-1
CED-3 homolog: Caspase-9
Together form the apoptosome (mammalian version of caspase holoenzyme)
Caspase holoenzyme/apoptosome = protease → degrades proteins → cell death
Loss of ced-3 or ced-4: no apoptosis
Loss of ced-9: uncontrolled cell death
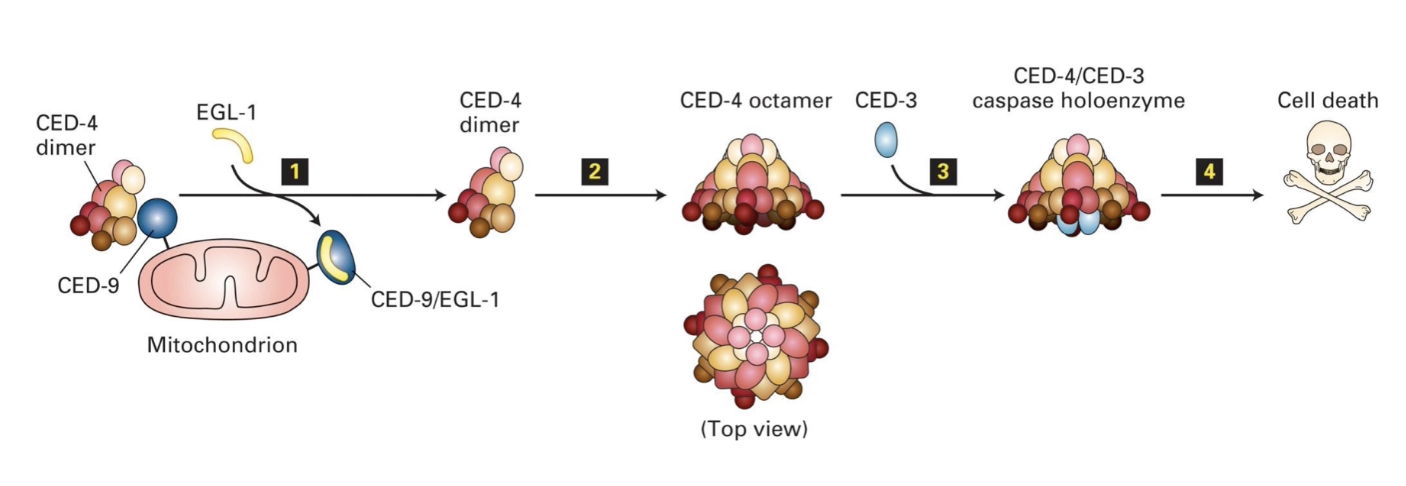
How does EGL-1 activate apoptosis in C. elegans?
CED-9 binds CED-4 dimers → keeps them inactive (prevents apoptosis)
EGL-1 binds CED-9 → releases CED-4
CED-4 joins CED-3 → forms caspase holoenzyme
Caspase activation → degrades cytosolic & nuclear proteins
Good model for mammalian apoptosome formation
What is the role of Bcl-2 in mammalian apoptosis?
Bcl-2 = mammalian homolog of CED-9
Location: Outer mitochondrial membrane
Function: Maintains low permeability → prevents apoptosis
Inactivation of Bcl-2:
Inactivation = pore formation → apoptosis
Leads to mitochondrial permeabilization
Pores release apoptotic factors (e.g., cytochrome c)
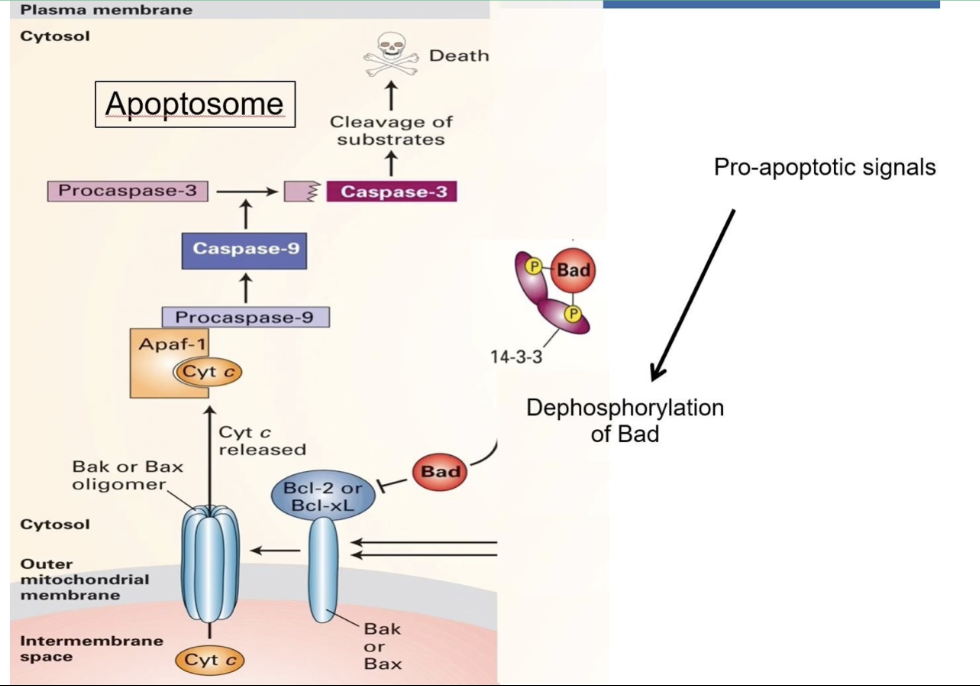
How is apoptosis regulated by Bcl-2, Bad, and Bax in mammalian cells?
Bad (pro-apoptotic signal)
Inactive when phosphorylated and bound to 14-3-3
Dephosphorylation → released from 14-3-3 → binds to Bcl-2 → blocks anti-apoptotic action
Bax (CED-9 family)
Activated when Bcl-2 is inhibited
Forms pores in mitochondrial membrane
Releases cytochrome c into cytosol
Cytochrome c
Triggers apoptosome formation
Essential for caspase activation → cell death
Triggers of apoptosis
Intrinsic: DNA damage, cell stress
Extrinsic: External death signals
How do trophic factors affect Bad and apoptosis?
Trophic factors → phosphorylate Bad → inhibit apoptosis
No trophic factors → Bad dephosphorylates → apoptosis
Kinase cascade keeps cell alive
Removal = death signal
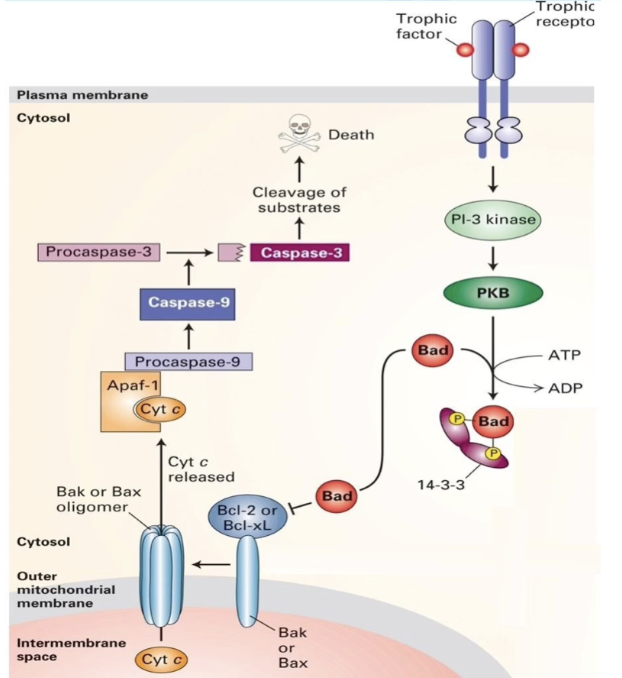
What are the key steps in the apoptotic pathway in response to the absence of trophic factors?
No trophic factor → Bad dephosphorylates
Bad inhibits Bcl-2/Bcl-XL→ Bax activates
Cytochrome c released
Apaf-1 + cytochrome c → caspase activation
Caspase = cysteine protease which targets proteins in nuclear lamina + cytoskeleton
Final apoptotic events
Chromatin condenses
Cytoplasm condenses
Nucleus fragments
Cell blebs → apoptotic bodies form
Apoptotic bodies phagocytosed by neighbors
Applied Lecture
Cancer
What is p53 and what does it do?
Tumor suppressor protein and transcription factor
Stops damaged cells from dividing → triggers apoptosis
Activates DNA repair and stress response pathways
Coordinates: cell cycle arrest, DNA repair, apoptosis, metabolism, anti-oxidant effects, etc.
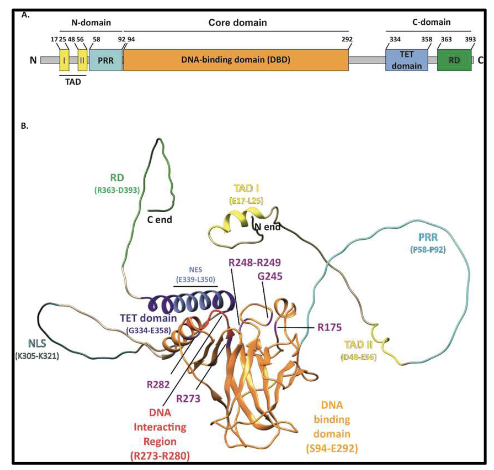
What are the key domains of p53 protein?
Tetramer of 4 chains (393 amino acids each)
N-terminal:
TAD I & II → bind transcription machinery & MDM2
DNA-binding domain (DBD)
Nuclear export signal (NES)
C-terminal:
Oligomerization domain (OD) → forms tetramer
Nuclear localization signals (NLS x3)
Second NES
NLS + NES → regulate nuclear-cytosolic shuttling
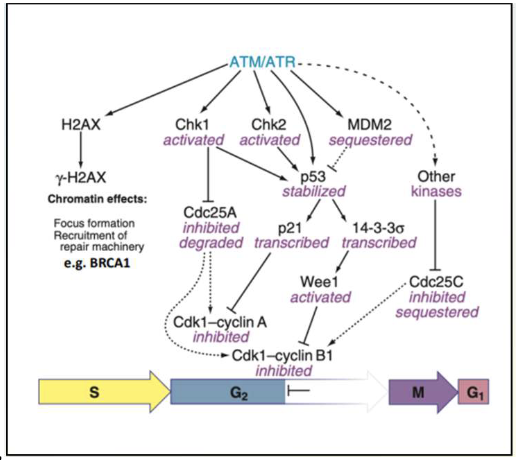
How does p53 protect the genome?
Cell cycle arrest:
Activates p21 → inhibits cyclin E/Cdk2 and cyclin D/Cdk4 → G1 arrest
Regulates G2/M via 14-3-3σ and cdc25C
DNA repair:
Activates multiple repair pathways: NER (nucleotide excision repair), BER (base excision repair), MMR (mismatch repair), NHEJ (non-homologous end joining)
Halts cycle to allow DNA repair
Constantly surveys for damage
What happens when p53 or other tumor suppressors are mutated?
Checkpoint failure → cells divide with DNA damage
Loss of mitotic control → indefinite division
p53 normally halts cycle or induces apoptosis
Mutated p53 found in >50% of cancers
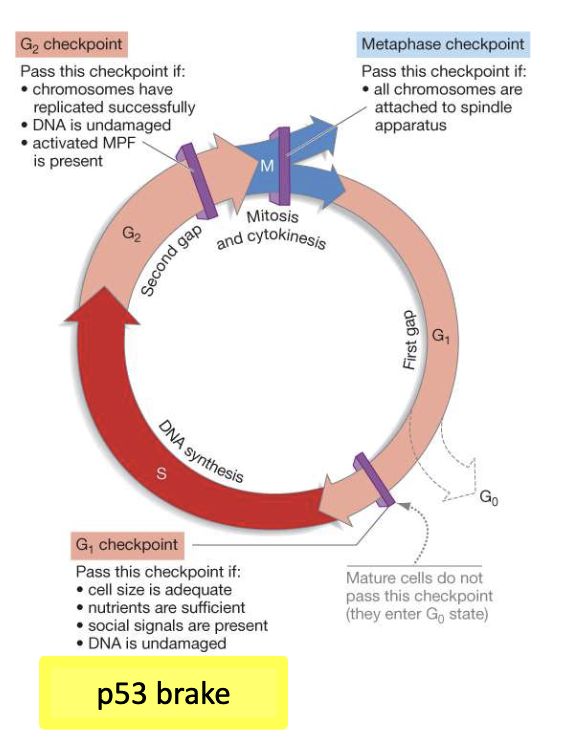
How is p53 involved in cancer development?
Mutated/deleted in ~50% of cancers
Remaining cancers → p53 pathway disrupted
Mutations let cells bypass checkpoints, resist apoptosis, proliferate unchecked
Targeting p53 is difficult but under study
Future therapies may combine p53 targeting + immunotherapy
What are challenges in targeting mutant p53?
Hard to find low-molecular-weight drug to bind mutant p53
Mutant p53 is nuclear → inaccessible to many drugs
Monoclonal antibodies don’t easily reach nucleus
Many different p53 mutations → unclear if one or multiple drugs needed
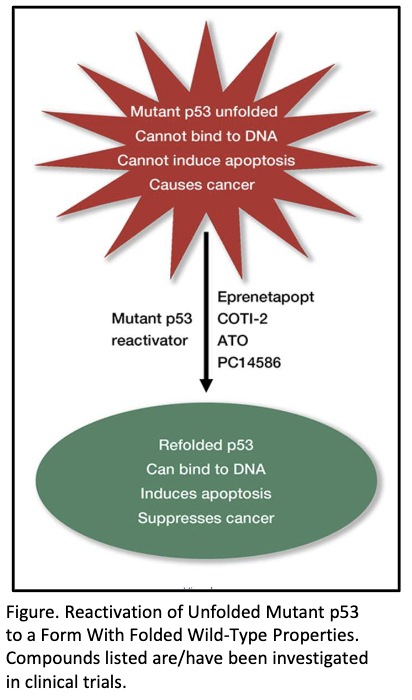
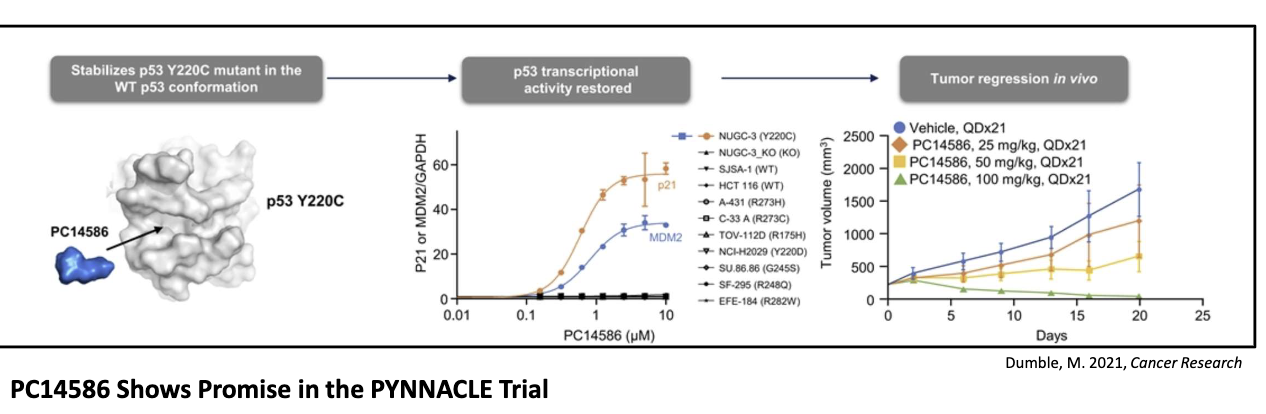
What is PC14586 and how does it work?
Small molecule corrector for Y220C p53 mutation
Restores wild-type shape and function
Selectively binds crevice formed by Y220C mutation
Found in ~1–2% of all p53 mutations across many tumors
Shows promise in early (PYNNACLE) trials
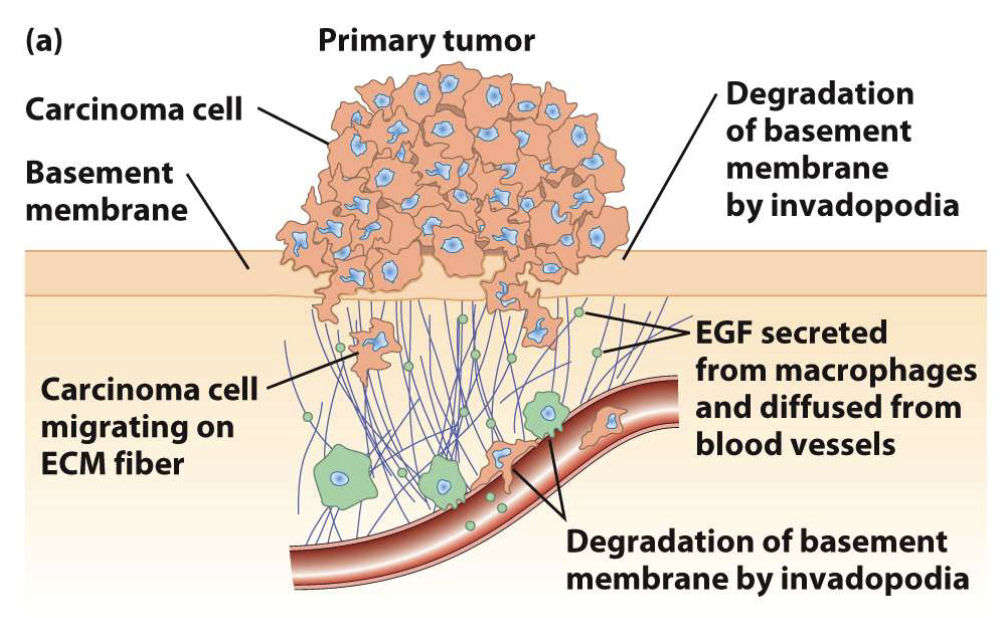
How do cancer cells spread (metastasize)?
Use invadopodia to breach basement membranes
Migrate to distant body sites
Example: breast carcinoma cells
How does apoptosis relate to cancer?
Cancer = uncontrolled cell growth + failure of apoptosis
Damaged cells avoid death → continue dividing
Leads to tumor formation (benign or malignant)