The Periodic Table and Atomic Mass (Learning Objectives)
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Periodic table has a number of ____ elements first published by ___ ___ in 1869. They are arranged by atomic ___. Vertical ____ representing groups and rows representing ___.
118; Dmitri Mendeleev; number; columns; periods
Be familiar with the first three periods. Try to name them
H, He, Li, Be (Beryllium) B, C, N, O, F, Ne, Na, Mg, Al (Aluminum), Si (Silicon), P (Phosphorous), S (Sulfur), Cl, Ar.
Group Classification:
Main group/representative: ___, ___, ___---___
Transition Metals: ___-___
Inner transition metals:
1, 2, 13-18
3-12
Lanthanides and Actinides
Elements within the same group have the same number of ___ in their valence shells, meaning they have similar chemical ____ and ____ types. (Not counting groups with a mix of metals/nonmetals)
electrons, properties, bonding
Group 1 is called the ___ metals. They are ___ (easy to cut), and look ____ that react violently with ___
alkali, soft, silvery, water
Group 2 is known as the ___ ___ ___. They are ___ reactive to water than group 1, they look ___, and reacts with ___
alkaline earth metals, less, shiny, O2
Group 16 is called the ___.
Group 17 are called ___. They are very ___ and ___ (eat away/damage)
Chalcogens
Halogens, reactive, corrosive
Group 18 are called the ___ ___. They are ____ and have very low chemical ____
noble gases, colorless, reactivity
Metals are typically ____ and ___-looking, have ___ mp/bp, and ___ conductor of heat/electricity.
hard, metallic, high, good
Nonmetals are typically ___, have ___ mp/bp, and are ___ conductors of heat/electricity
soft, low, poor
Metalloids are ___-___ properties.
In-between
Metallic trend is how easy an atom will give up an ____. Their metallic character will ___ down a group and ___ down a row. (Extra points, explain why this is)
electron, increase, decrease
Increase down a group: they have bigger shells (further electrons), so easy to take away
Decrease down a row: More protons in a nucleus, same valence shell, so harder to take
Atomic mass: 1 amu (aka ___) is exactly ___ of a carbon-12 atom.
Da, 1/12
1 amu = ____ kg
1.661 × 10^(-27)
1 amu = ____ g
1.661 × 10^(-24)
Atomic mass refers to the mass of a single atom. But on the periodic table, it is the ___ ___ for all naturally occurring ___ of an element.
weighted average, isotopes
(Need to do the math for this one)
Carbon has 2 isotopes: 12C (12.000 amu, 98.89% abundance) and 13C (13.003 amu, 1.11% abundance). Find the average atomic mass
(12.000 amu x 0.9889) + (13.003 amu x 0.0111) = 12.011 amu
Natural carbon is composed of two isotopes, ¹²C (12.000 amu) and ¹³C (13.003 amu). The average atomic mass of carbon is 12.011 amu. Calculate the percent abundance of each isotope.
12.000x + 13.003y = 12.011 amu
x+y=1
then solve by substitution
1 Mole = ____ atoms/molecules/ions. This hidden number is also called ___’s number
6.022 × 10^(23); Avogadro’s
If H2O is roughly 18 amu, then that means it’s also 18 ___/___ so for every 18 ___, there is 1 mole of H2O, which means there are ____ molecules of H2O. However, there are ___ moles of H and ___ mole of O.
g/mol, grams, 6.022 × 10^(23), 2, 1
Molecular mass is found by ___ all amus on the periodic table. For instance, in H2O, what would the molecular mass be?
adding
2 H’s, 1 O —→ 2(1amu) + 16 amu = 18amu
Molar mass is found by calculating the ____ mass and then just changing atomic mass units to ___. For instance, what is 18 amu in molar mass?
molecular, g/mole, 18g/mole
Proof that 1 amu per particle = 1 grams per mole (just review, no need to know it)
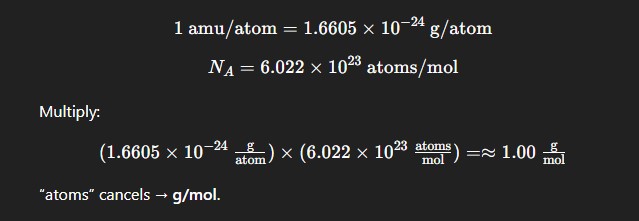
If I had 36 grams of H2O, how many moles do I have?
Molecular mass = 18 amu = 18 g / mole
36 grams divided by 18 g/mole = 2.0 moles of H2O
If I had 2.0 moles of H2O, how many particles do I have?
Avogadro’s number: 6.022 × 10^(23) particles / mole
2.0 moles multiplied by 6.022 × 10^(23) particles / mole = 12.0 × 10^(23) = 1.2 × 10^(24) molecules of H2O
If I had 3.6 × 10^(23) particles of O, how many moles do I have?
Avogadro’s number: 6.022 × 10^(23) particles per mole
3.6 × 10^(23) / 6.022 × 10^(23) = 0.498 Moles
If I had 0.498 moles of O, what is its mass in grams?
O = 16 amu = 16 g / mol
0.498 moles (16 g / mol) = 7.97 g
To go from mass to moles, you take the mass of the substance given in ___, find the ____ mass of the substance in amu, convert into ____, and then ___ the mass given by the conversion.
Ex: 16g of O is how many moles?
grams, molecular, g/mol, divide
16g is given, O = 16amu = 16 g/mol —> 16g / 16 g/mol = 1 mole.
On the periodic table, metals are to the ___, nonmetals are to the ___, and metalloids are along a ___
left, right, zigzag (staircase)
Periodicity of the periodic table:
Repeating ___ of element ____ when ordered by ___ ___. Properties include ____ character, atomic ___, and ____
patterns, properties, atomic number, metallic, radius (decreases right to left and increases from top to bottom), electronegativity (increases right to left and bottom to top)