C1.1 Solids, liquids and gases
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
5 Terms
State the distinguishing properties of solids, liquids and gases/
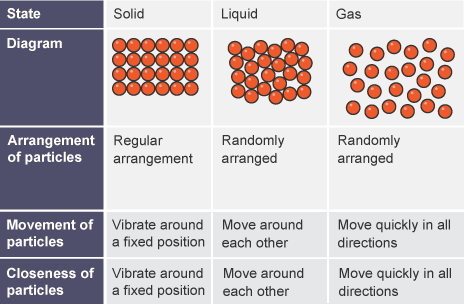
Describe the structure of solids, liquids and gases in terms of particle separation, arrangement and motion
Solids: fixed shape, fixed volume, strong intermolecular forces, particles are very close together, vibrate around a fixed position
Liquids: changes shape to fit container, fixed volume, weaker intermolecular forces than solids, particles are not as packed in solids but close together, move around each other randomly
Gases: no fixed shape, changes volume to fix container, weakest attractive forces, particles far apart and move quickly
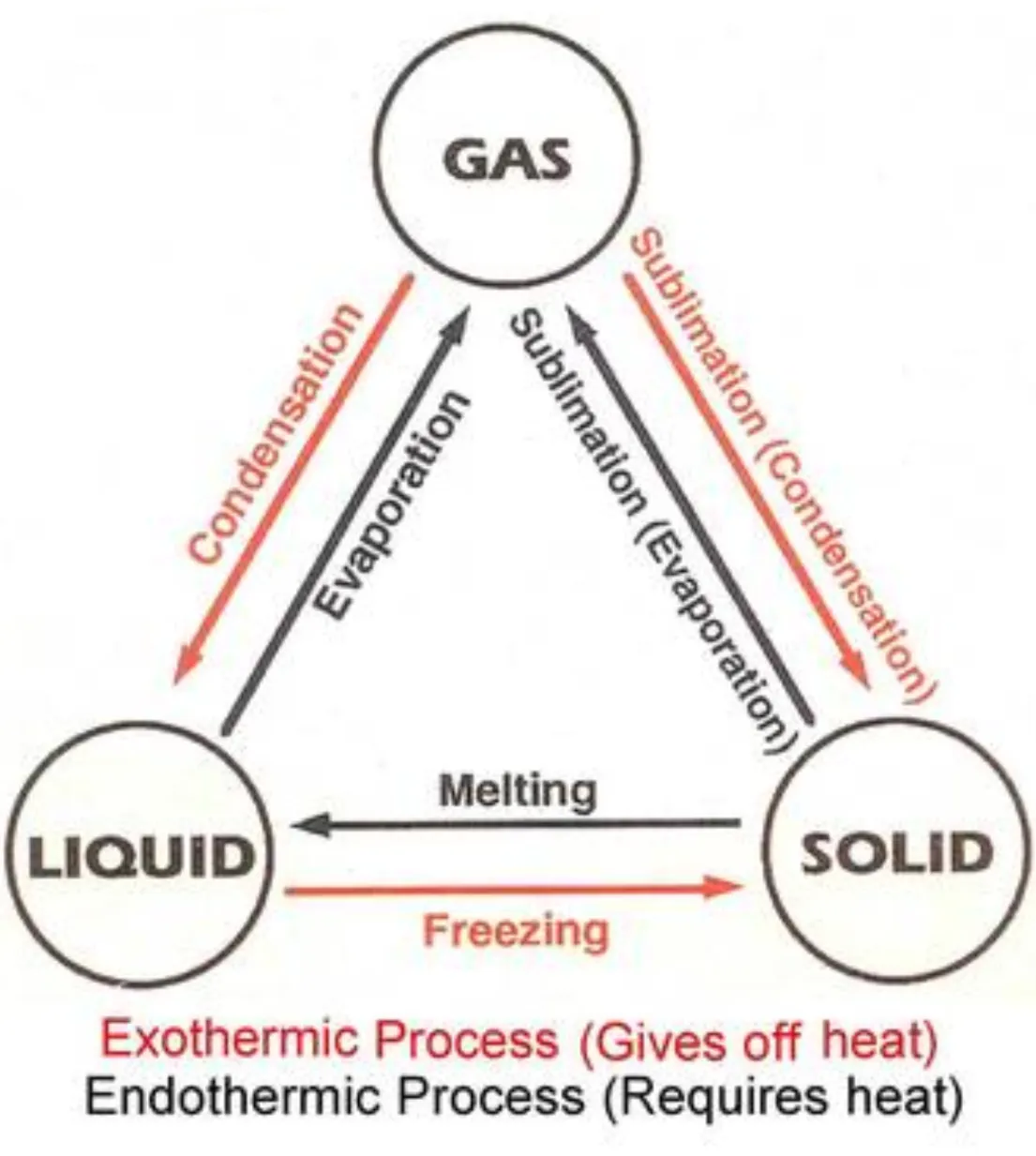
Describe changes of state in terms of melting, boiling, evaporating, freezing and condensing
Solid to liquid: freezing - exothermic
Liquid to solid: melting - endothermic
Liquid to gas: evaporation (at any temperature and on the surface of the liquid) - exothermic
Liquid to gas: boiling (at a specific temperature and throughout the liquid) - endothermic
Gas to liquid: condensation - exothermic
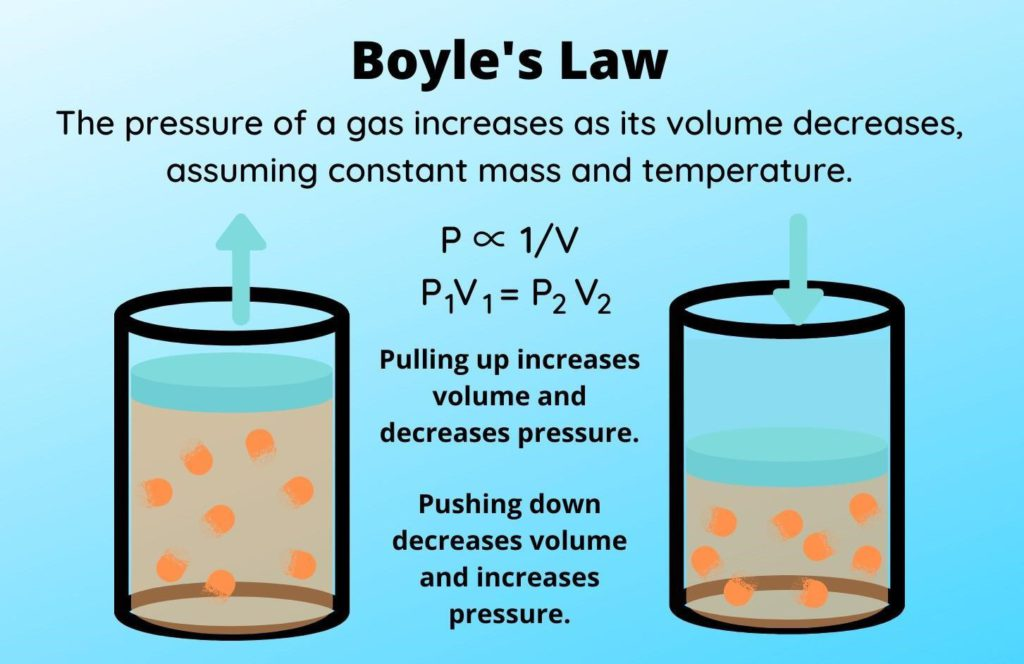
Describe the effects of temperature and pressure on the volume of a gas/
Explain, in terms of kinetic particle theory, the effects of temperature and pressure on the volume of a gas
Charles Law: if pressure is held constant, temperature and volume of a gas are directly proportional
Higher temperature = more kinetic energy = hits walls of container more = volume expands
Boyles Law: at constant temperature, pressure and volume are inversely proportional
Higher volume = particles have to travel more to collide with container walls = less pressure applied
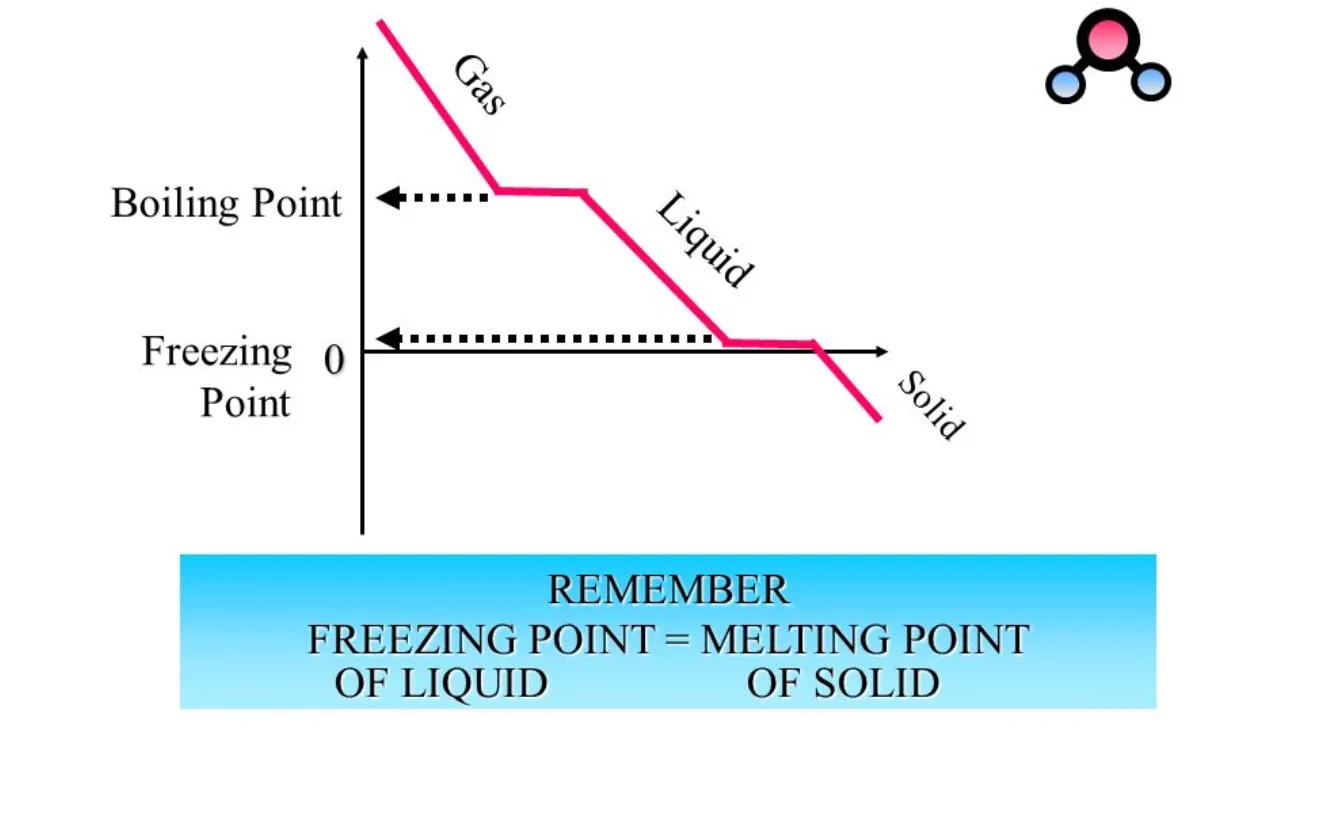
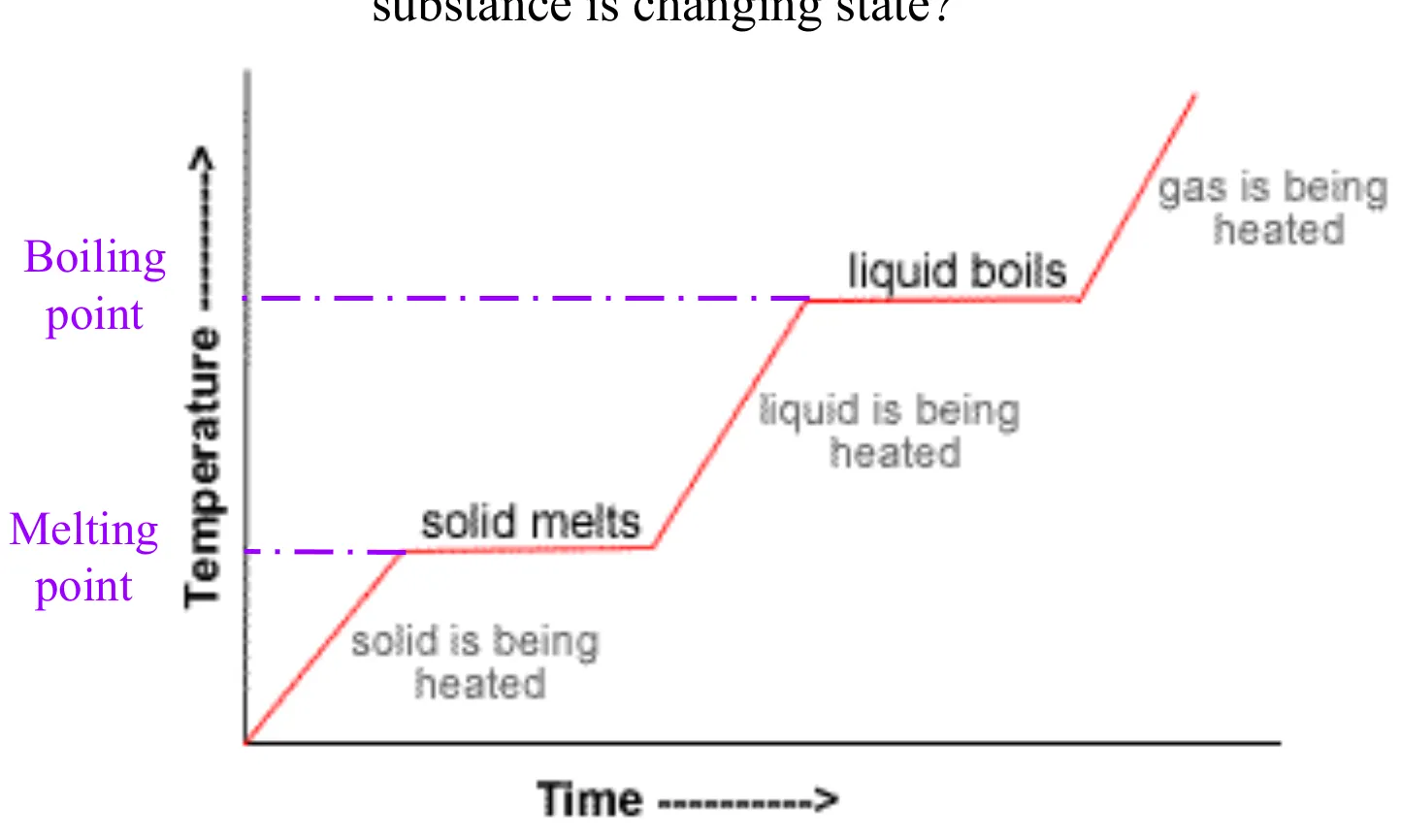
Explain changes of state in a heating curve in terms of kinetic particle theory
Solid to liquid (melting): energy is being absorbed (endothermic process). The energy increases the potential energy of the particles and weakens/overcomes the intermolecular forces that hold the particles in fixed positions, allowing the particles to slide over each other.
Liquid to gas (boiling): energy is being absorbed (endothermic process). The energy increases potential energy of the particles and weakens/overcomes the intermolecular forces that hold the particles close together, allowing the particles to move far apart freely.
Clarification: during the phase changes (horizontal), temperature does not change. Only in the slopes (ex. when the solid is being heated) is when temperature changes.
Explain changes of state in a cooling curve in terms of kinetic particle theory
Gas to liquid (condensation): particles release energy (exothermic process). Release of energy decreases kinetic energy of particles and they can no longer overcome intermolecular forces, so the particles get closer together and move to form a liquid.
Liquid to solid (freezing): particles release energy (exothermic process). Release of energy decreases kinetic energy of particles and they can no longer overcome intermolecular forces, so the particles get closer together in a fixed position to form a solid.
Clarification: during the phase changes (horizontal), temperature does not change. Only in the slopes (ex. when the gas is being cooled) is when temperature changes.