AP Chem Unit 1
1/114
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
115 Terms
Observe
Macroscopic
Imagine
Particulate
Represent
Symbolic
How we study nature
Scientific Method
Observation
Collecting data
Qualitative
Descriptive characteristics
Quantitative
Number and Unit
Hypothesis
making predictions
Experiments
testing hypothesis
Theory
accepted explanation of WHY nature behaves a certain way
Law
FACTS summarizing HOW nature behaves
2 Types of Pure substances
elements and compounds
Elements are made up of
Atoms (elemental symbol)
Compounds are made up of
Molecules (chemical formula)
How are Elements separated
Only able to be separated by Nuclear Means
How are Compounds separated
Only able to be separated by Chemical Means
Types of Elements
Monoatomic and Diatomic
Types of Compounds
Covalent (share electrons) and Ionic (transfer electrons)
Diatomic Elements
H2, N2, O2, F2, Cl2, Br2, I2
Monoatomic Elements
composed of single atoms not bonded to each other
Covalent Compounds are made up of
All Nonmetals
Ionic Compounds are made up of
A Metal and a Nonmetal
Covalent Compounds make
Molecules
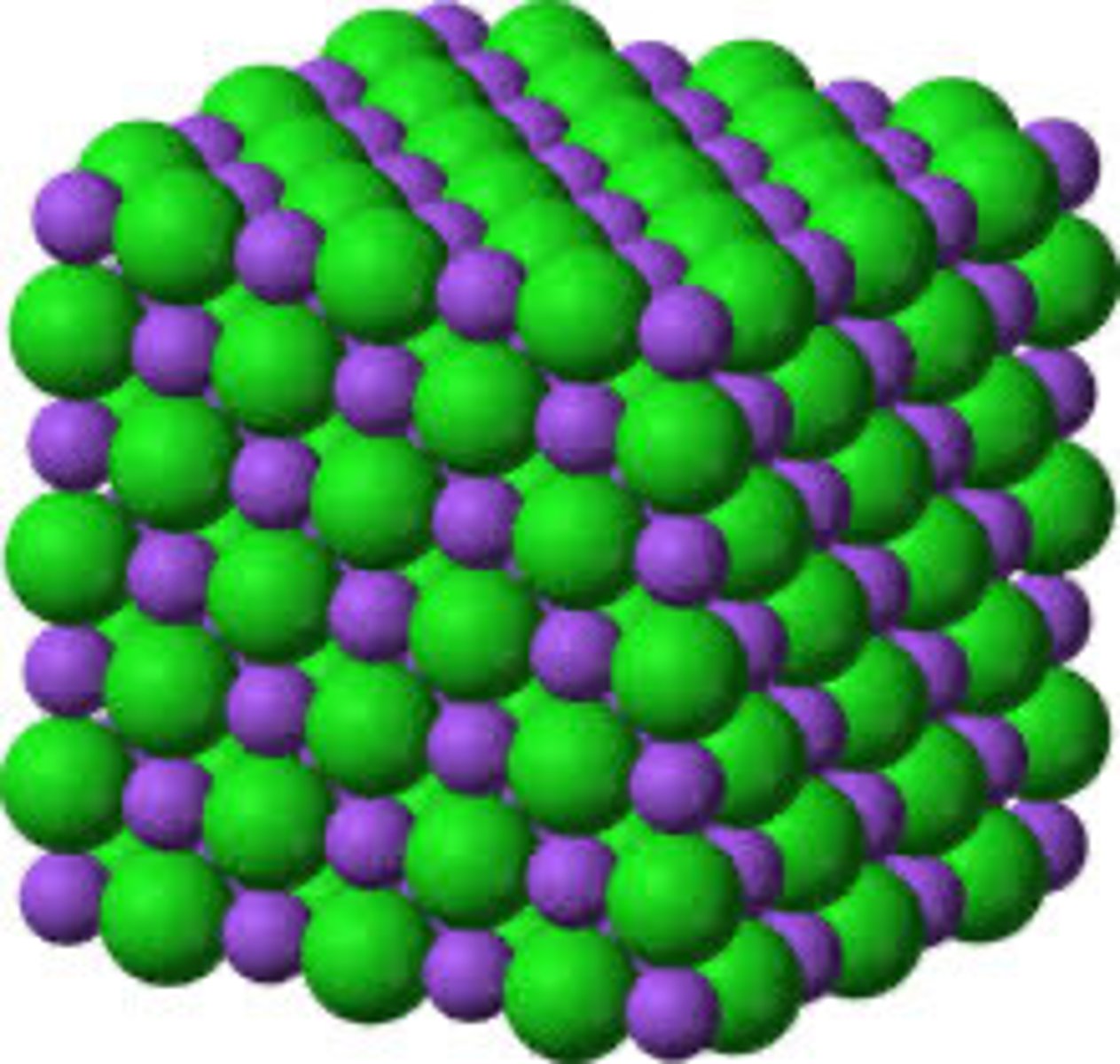
Ionic Compounds make
Crystal Lattices
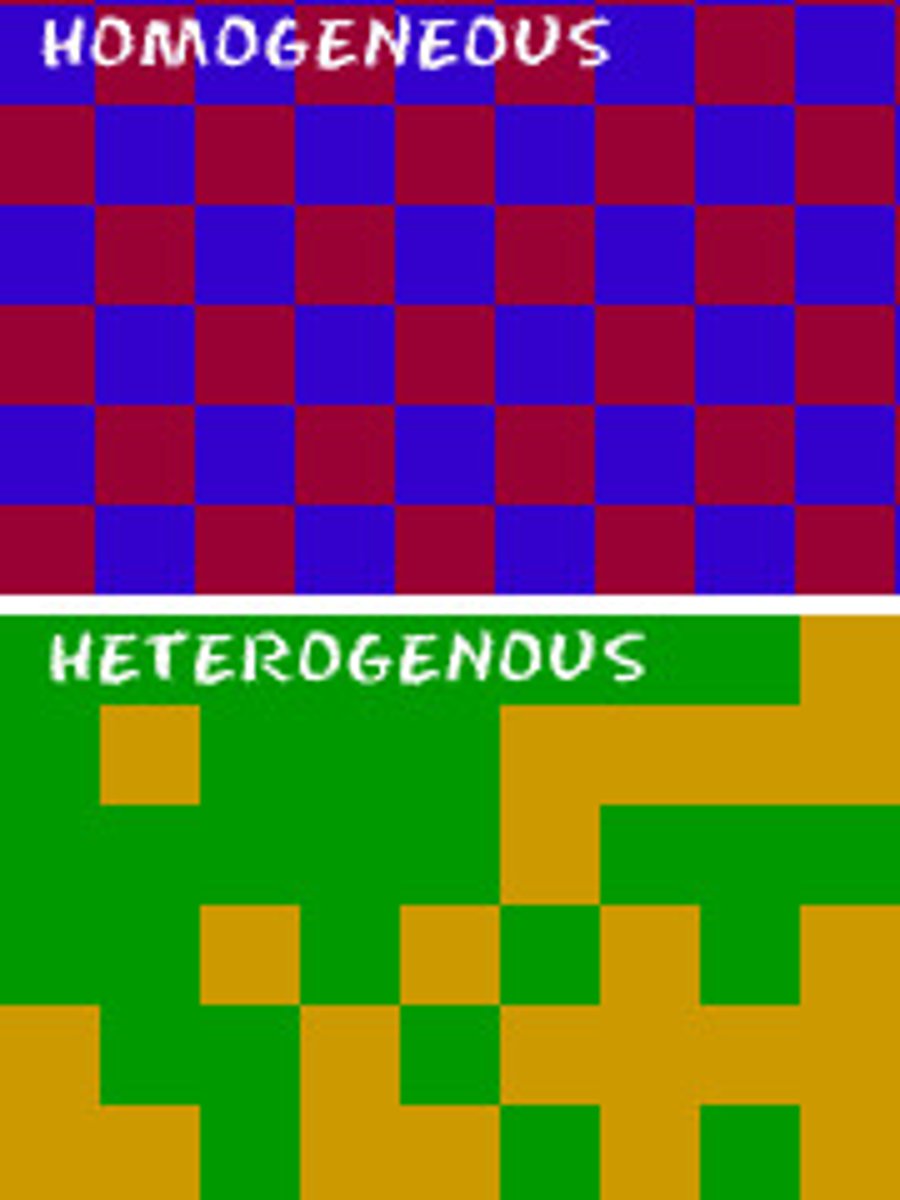
Two Types of Mixtures
Homogenous and Heterogenous
Homogenous Mixture
A uniform mixture
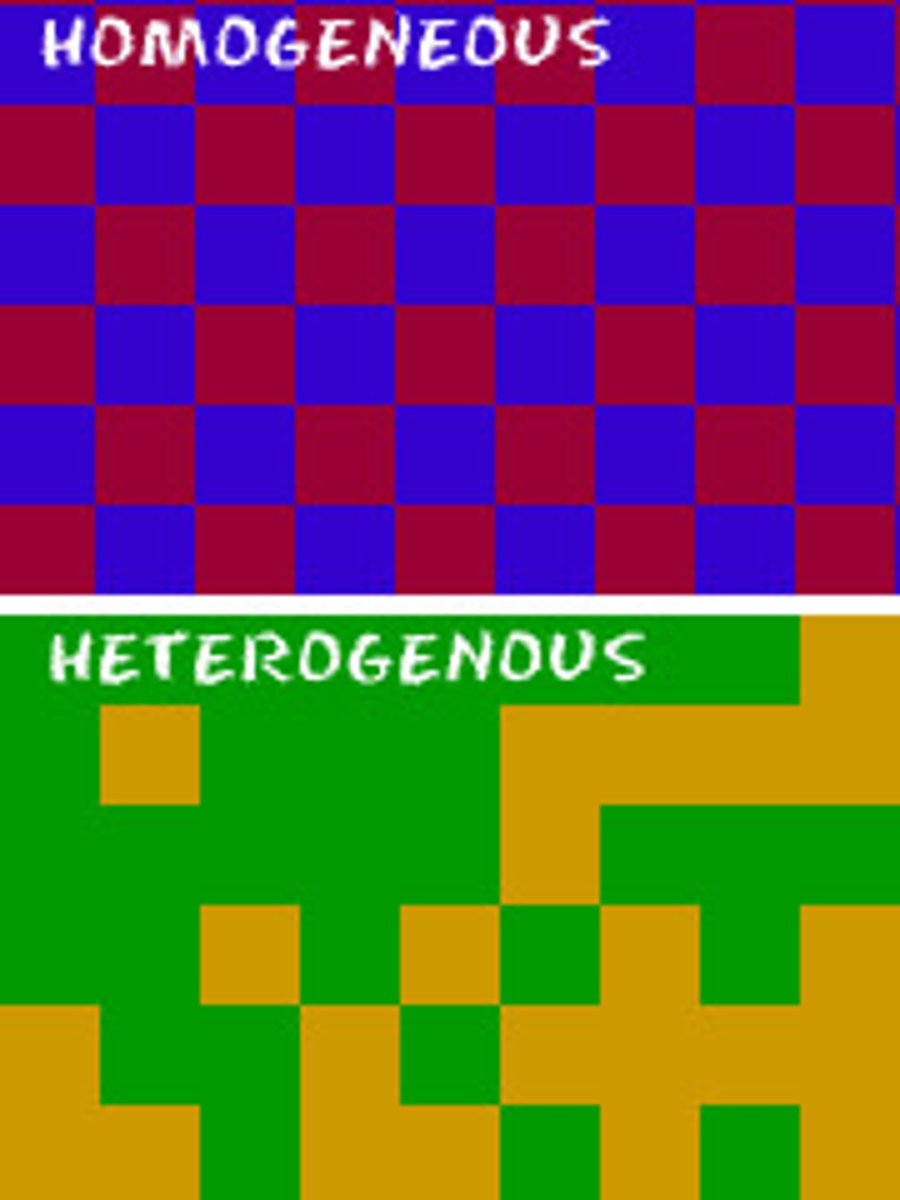
Heterogenous Mixture
A nonuniform mixture
Properties of Matter
physical and chemical
Physical Properties
Observed without altering the chemical makeup
Chemical Properties
Once observed chemical makeup changes
Intensive
Independent of the amount of a substance
Extensive
Depends on the amount of substance
Types of Intensive Properties
melting point, boiling point, density, ability to conduct electricity, ability to transfer energy as heat
Types of Extensive Properties
volume, mass, amount of energy in a substance
Types of Chemical Properties
corrosiveness, flammability, acidity, toxicity
Centrifuge and Decanting
solid & liquid (insoluable)
Centrifuge and Decanting is
Qualitative
Distillation
Volatile liquid vaporizes first (homogenous)
Filtration
Solid & liquid (insoluble)
Gravity Filtration is
Quantitative
Vacuum Filtration is
Qualitative
Two Types of Filtration
Gravity Filtration and Vacuum Filtration
Evaporation Crystallization
Soluble solid in liquid
Chromatography
Different Colored Substances
Accuracy
how close a measurement is to the true value
Precision
The degree to which repeated measurements show the same result.
% error
theoretical-experimental/theoretical x 100
% difference
trial 1-trial2/average x 100
Law of Definite Proportions
Atoms combine in whole # ratios
Law of Multiple Proportions
Atoms can combine in different ratios
% Composition
mass of part/mass of whole x 100
Empirical Formula
Lowest whole number of atoms ratio
Steps for Empirical Formula
1. If the percent is given, assume a 100 g sample so that percent is equal in grams
2. Change Grams to moles
3. Get the smallest whole number ration— divide each result from step two by the smallest result
4. If necessary (like if it is a .5 or something), multiply by whole number
5. Write the empirical formula
Molecular Formula
actual ratio of atoms
Franklin
Opposites Attract
Lavoisier
Law of multiple and definite proportions
Milkan
Mass of the electron
Dalton
atomic theory
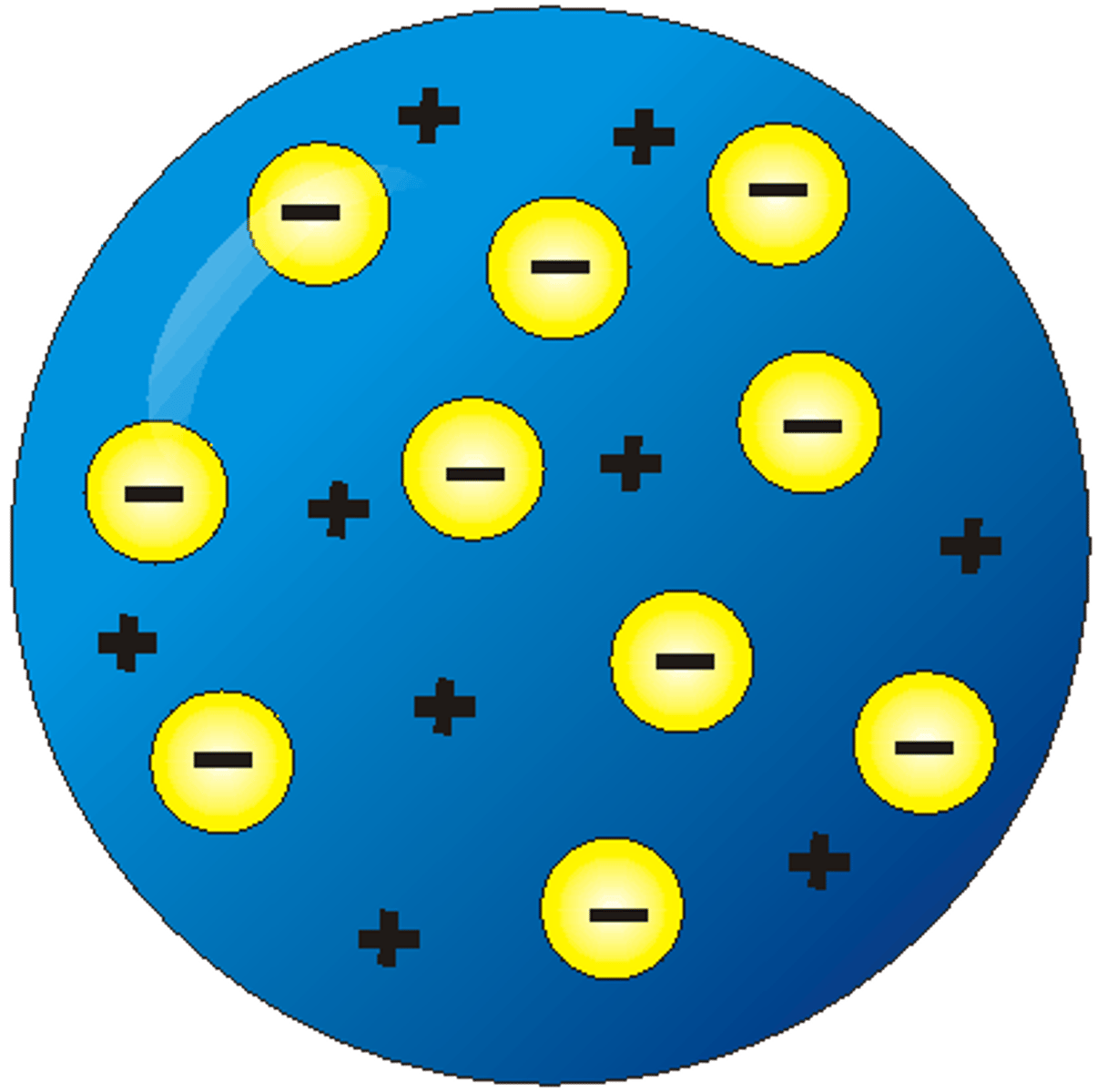
Thompson
Plum Pudding and Catharay Tube
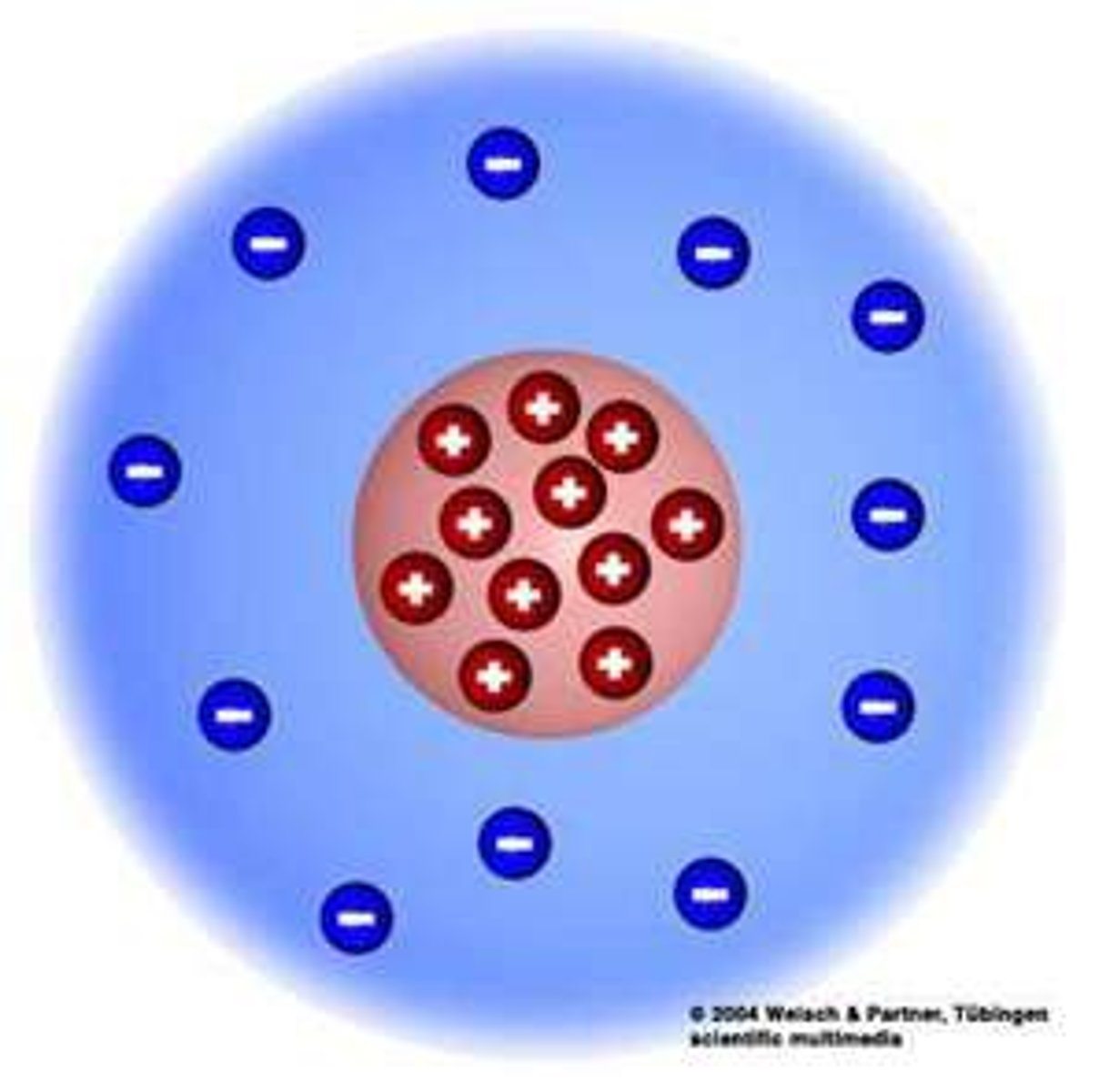
Rutherford
Gold Foil and Nucleus
Bohr
Energy Levels

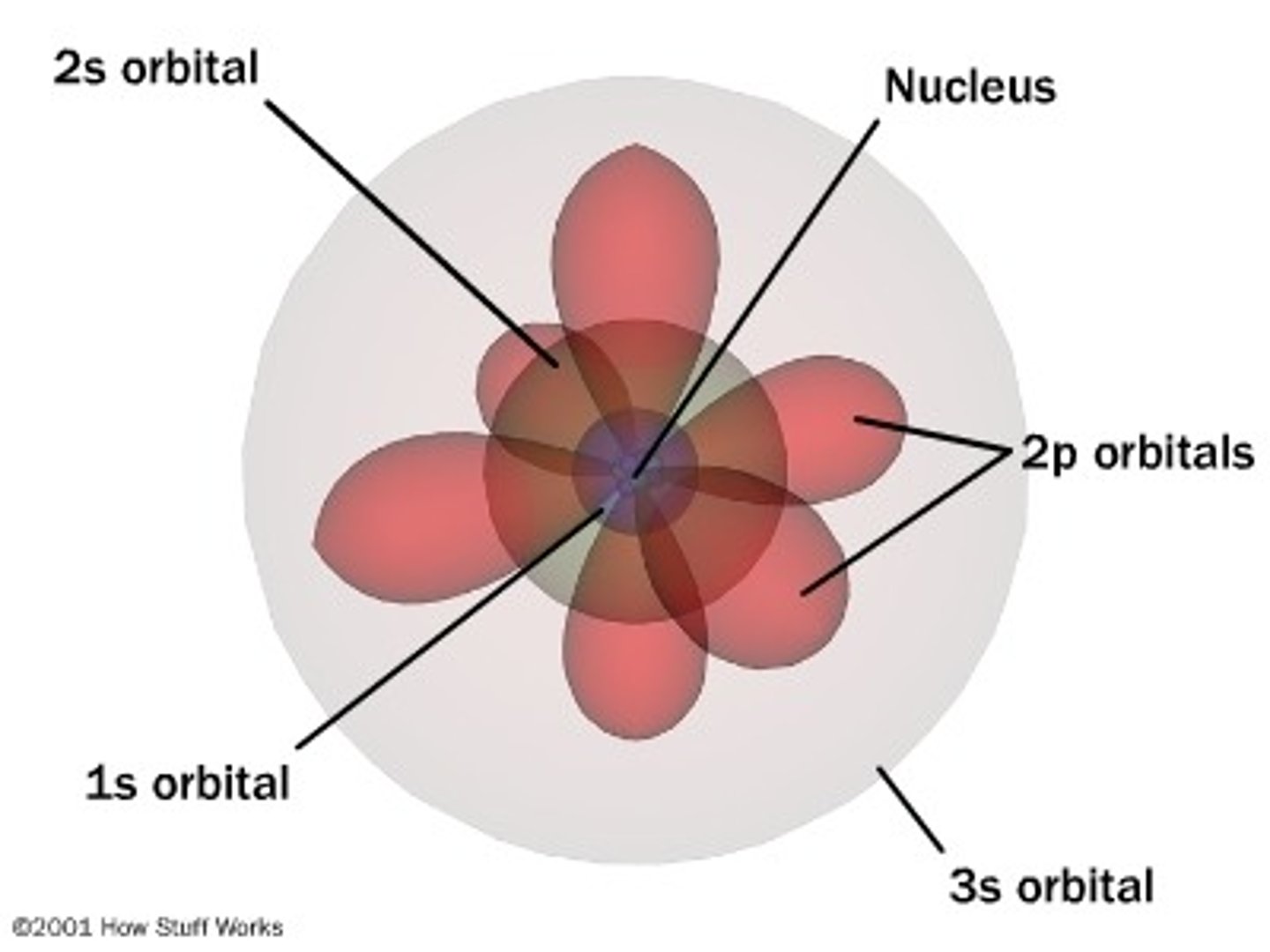
Schrodinger
Electron Cloud
Counting Subatomic Particles
Mass # over Atomic #
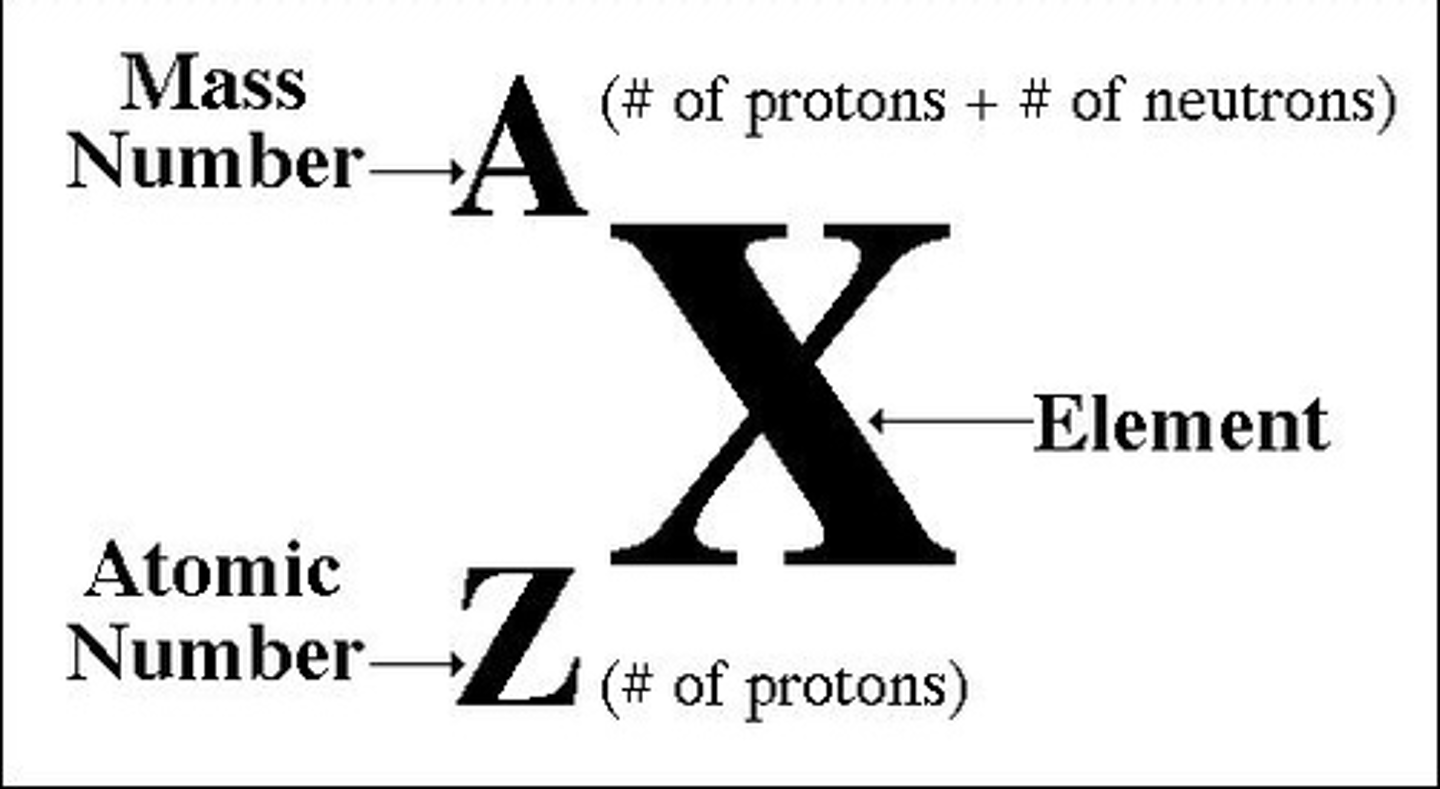
Mass #
# of protons + # of neutrons
Atomic #
# of protons
For any given element, the size of an isotope with more neutrons is larger than one with fewer neutrons
FALSE - an e- determines the size
For a given element, the size of an atom is the same for all of the element's isotopes
TRUE - Masses would be different not size
Atomic Mass
Average mass of all the isotopes of that element
Atomic Mass Steps
1. Convert % abundance to decimal
2. Multiply decimal by mass of each atom
3. Add them together
Oxidation Numbers Rule 1
An element in its natural form (not combined with another element) has an oxidation number of 0
Oxidation Numbers Rule 2
Monoatomic metal ions in Groups 1 & 2 have oxidation # equal to their charge
Oxidation Numbers Rule 3
Oxygen has an oxidation # of -2; Except when it's in a peroxide (Metal O2) where each oxygen is -1
Oxidation Numbers Rule 4
Hydrogen has an oxidation # of +1; Except in metal hydrides (MxHy), then its -1
Oxidation Numbers Rule 5
Halogens are -1; Except when combined w/ O or F
Oxidation Numbers Rule 6
Sum of all oxidation #'s must add to zero or the charge of the ion
Prefix 1
Mono
Prefix 2
Di
Prefix 3
Tri
Prefix 4
Tetra
Prefix 5
Penta
Prefix 6
Hexa
Prefix 7
Hepta
Prefix 8
Octa
Prefix 9
Nona
Prefix 10
Deca
Phosphate
PO4 3-
Sulfate
SO4 2-
Chlorate
ClO3 1-
Carbonate
CO3 2-
Nitrate
NO3 1-
Hydroxide
OH 1-
Acetate
C2H3O2 1-
Polyatomic Anion Name
Per____ate
____ate
____ite
Hypo____ite
____ide
Acid Name
Per____ic acid
____ic acid
____ous acid
Hypo____ous acid
Hydro____ic acid
5 Steps to Limiting Reactant Problems
1. Write BCE
2. Determine the moles of each reactant initial (goes in ICE)
3. Determine the number of moles that react (change) with the mole ratio.
4. Determine the limiting reactant by setting initial - change = 0 for both reactants. Whichever is the smallest x is the Limiting Reactant.
5. Use the limiting reactant to answer the question. Use the excess reactant to determine whats leftover.
Making a Solution from a Solid
1. Mass the amount of solid
2. Fill the size volumetric flask 1/3 to 1/2 full with distilled water
3. Transfer the Solid to a Volumetric Flask
4. Swirl until dissolved (add more water if necessary)
5. Fill the flask with distilled water until the bottom of the meniscus touches the etched mark
6. Cap & Invert or Pour into a Beaker and Parafilm
Making a Solution by Dilution (from Stock)
1. Determine the molarity needed, amount needed, and tock concentration (use to determine the pipet size)
2. Obtain Pipet and Volumetric Flask
3. Pipet stock into a volumetric flask, be sure to touch the tip of the side
4. Fill the rest of the flask with distilled water until the bottom of the meniscus touches the etched mark
5. Cap & Invert or Pour into a Beaker and Parafilm
Making a Solution by Dilution FORMULA
M1 (Thing in pipet or stock) V1 = M2 V2 (amount of diluted)
Strong Electrolytes
Dissociates completely when dissolved in water
Strong Electrolytes Examples
Strong Ionic Compounds (salts
Strong Bases
Strong Acids