Aromaticity & Benzene Reactions
0.0(0)
0.0(0)
Card Sorting
1/20
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
1
New cards
aromaticity rules
cyclic and conjugated, none are SP2
4n+2 rule
2
New cards
a compound is acidic if
deprotonation makes it aromatic
3
New cards
if loss of leaving group makes a compound aromatic, then the compound is more inclined to undergo
Sn1 reactions
4
New cards
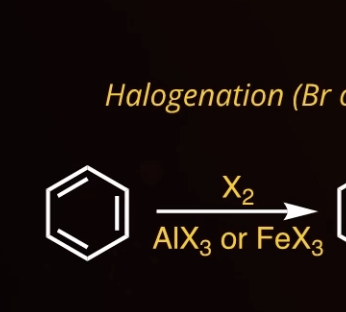
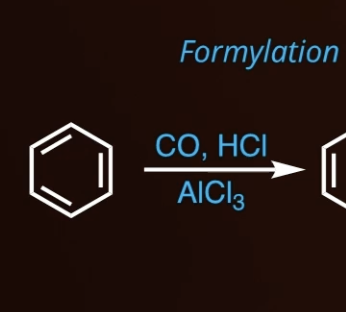
5
New cards
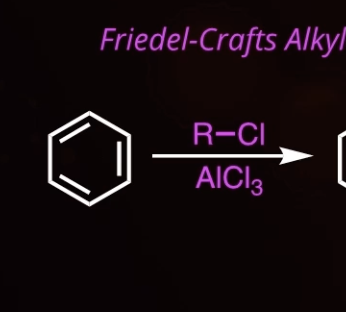
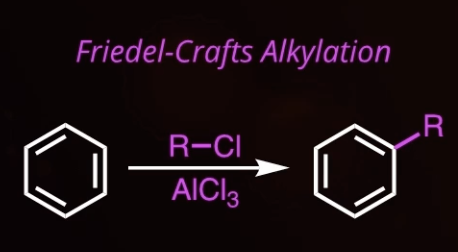
6
New cards
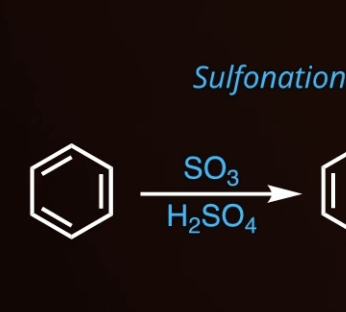
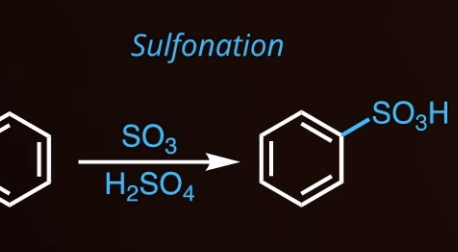
7
New cards
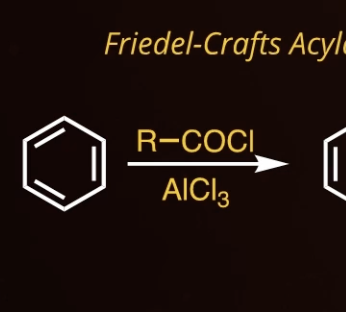
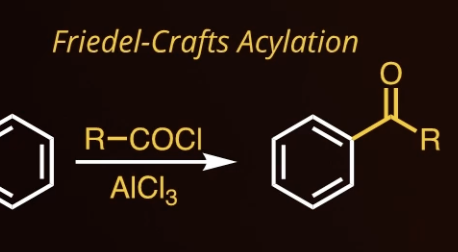
8
New cards
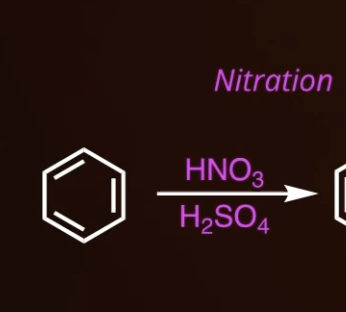
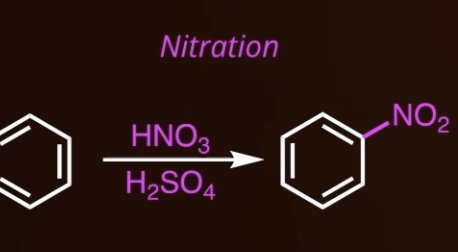
9
New cards
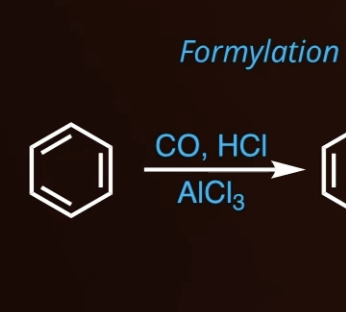
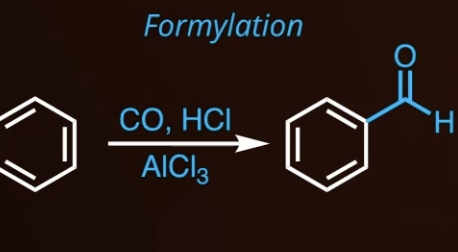
10
New cards
meta directors
deactivating
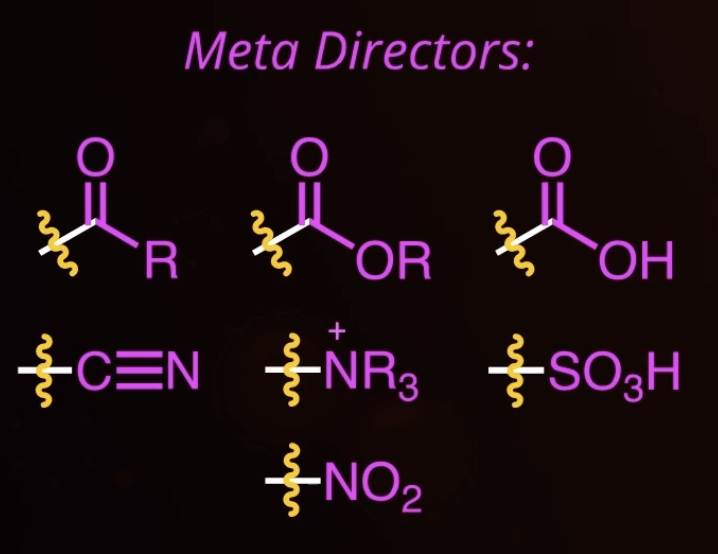
11
New cards
ortho/para directors
F, CL, Br, I
activating
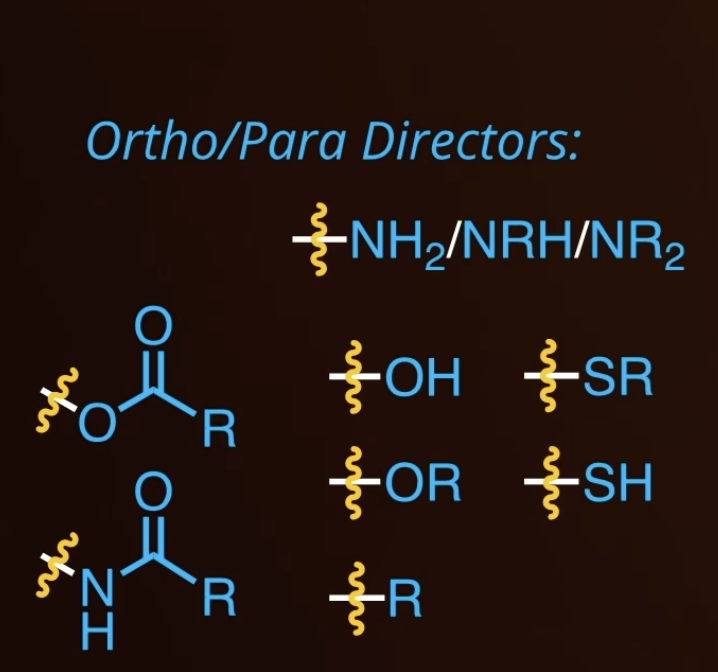
12
New cards
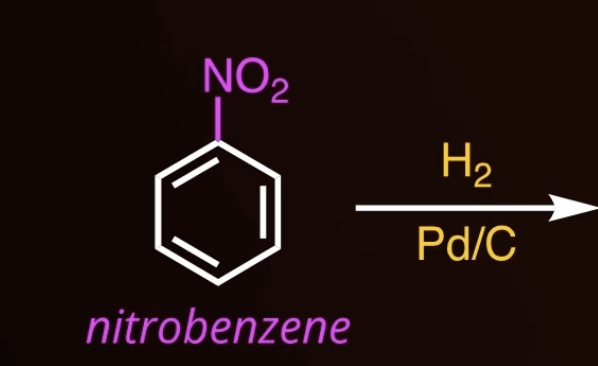
reduction of a nitro group
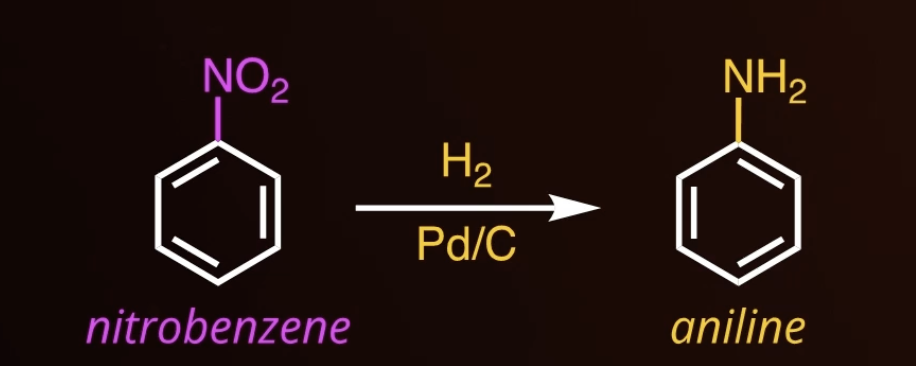
13
New cards
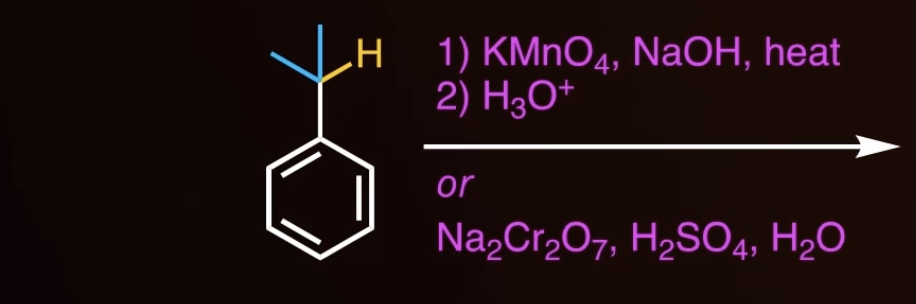
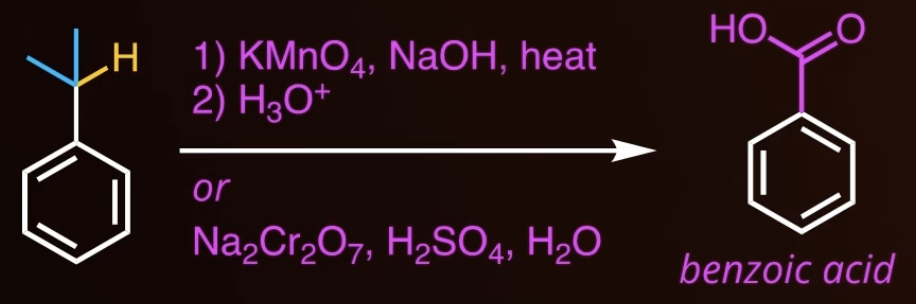
side chain reaction
doesnt work with tertiary!
14
New cards
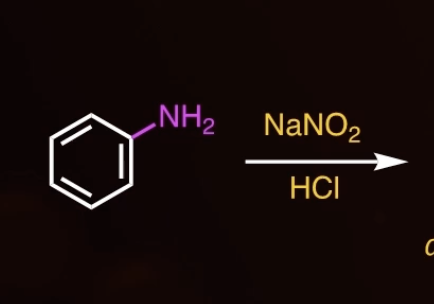
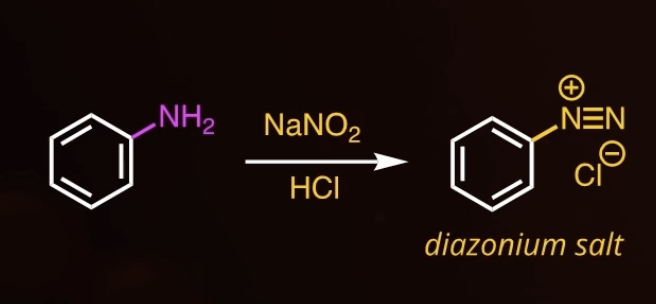
DIAZONIUM SALT
15
New cards

H3PO2
benzene ring
16
New cards

KI
I
17
New cards
HBF4
F
18
New cards
H3O+, heat
OH
19
New cards
CuCN
CN
20
New cards
CuBr
Br
21
New cards
CuCl
Cl