Chemistry Unit 4 AOS 1
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
Organic Compounds
Carbon-based compounds with diverse structures and functions.
Molecular Formula
Shows number and type of atoms in a compound.
Structural Formula
Illustrates the arrangement of atoms in a molecule.
Skeletal Formula
Simplified representation of organic compounds.
Isomers
Compounds with the same formula but different structures.
Hydrocarbons
Compounds consisting only of carbon and hydrogen.
Alkanes
Saturated hydrocarbons with single carbon bonds.
Alkenes
Unsaturated hydrocarbons with at least one double bond.
Cyclohexane
Cyclic alkane with six carbon atoms.
Degree of Unsaturation
Indicates number of rings and double bonds present.
Haloalkanes
Alkanes with halogen substituents like F, Cl, Br, I.
Alcohols
Organic compounds with hydroxyl (-OH) functional group.
Primary Alcohols
One alkyl group attached to carbon with -OH.
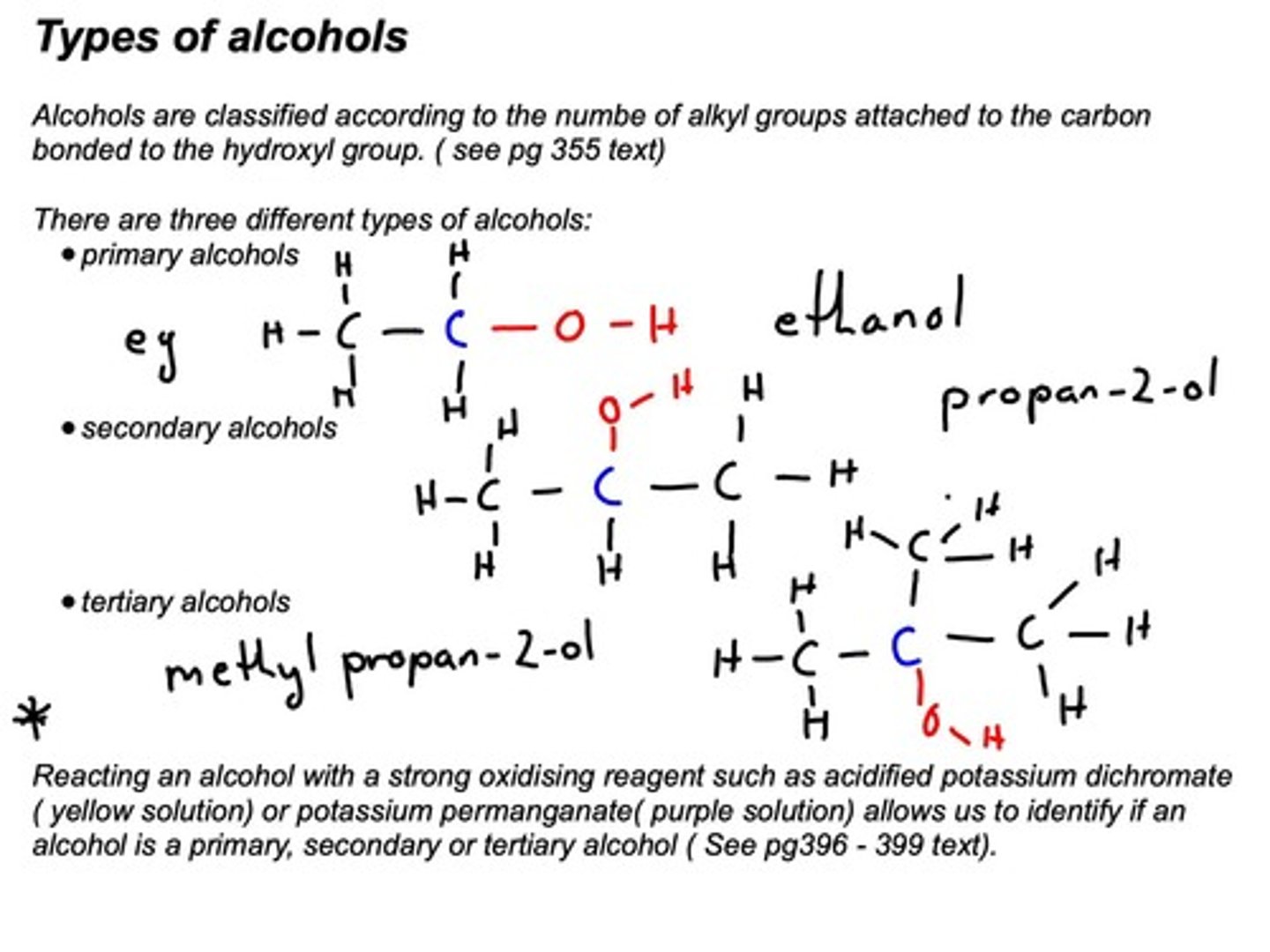
Secondary Alcohols
Two alkyl groups attached to carbon with -OH.
Tertiary Alcohols
Three alkyl groups attached to carbon with -OH.
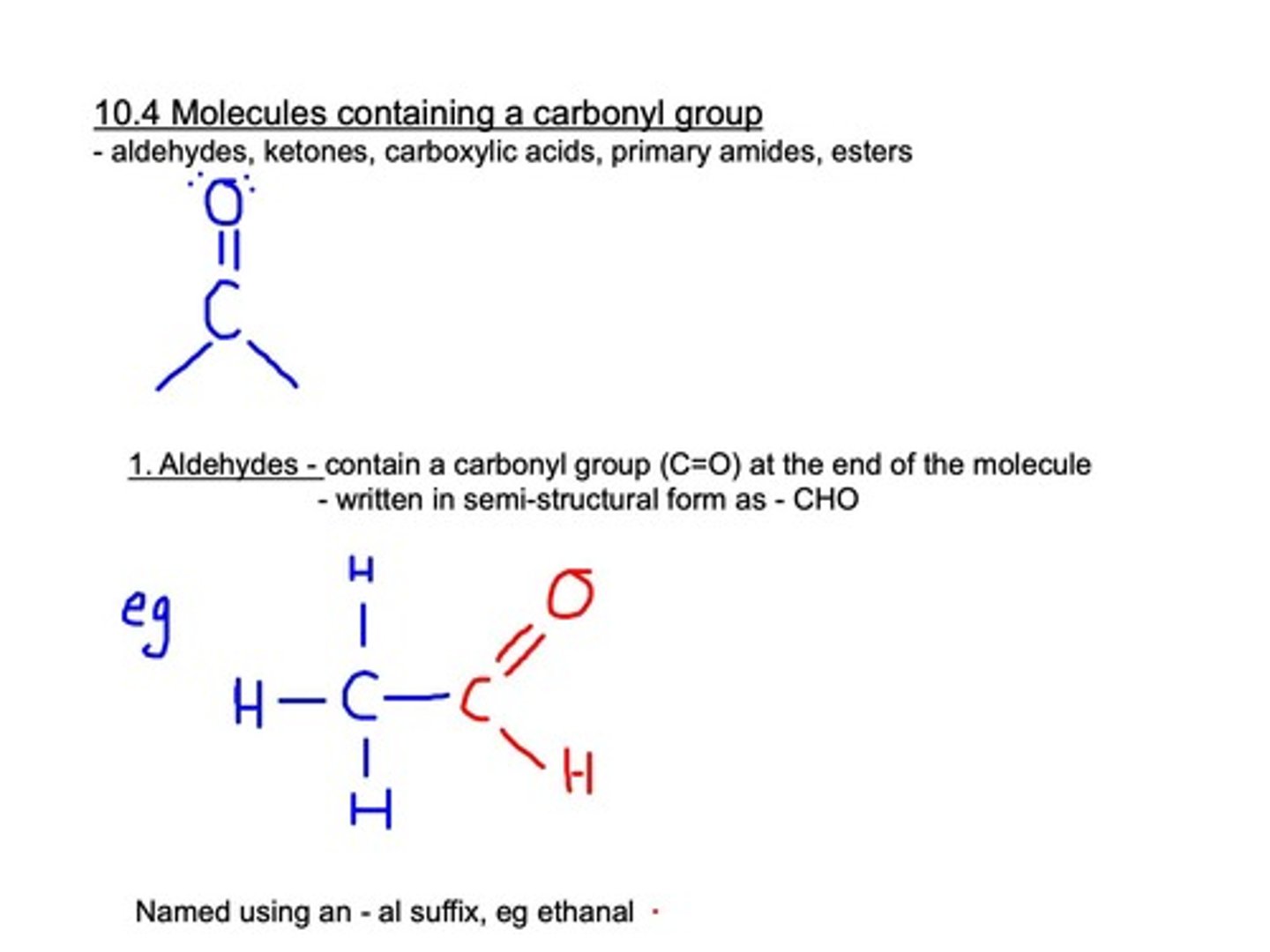
Carbonyl Group
Functional group with a carbon double-bonded to oxygen.
Aldehydes
Contain carbonyl (CHO) group at the end of the molecule.
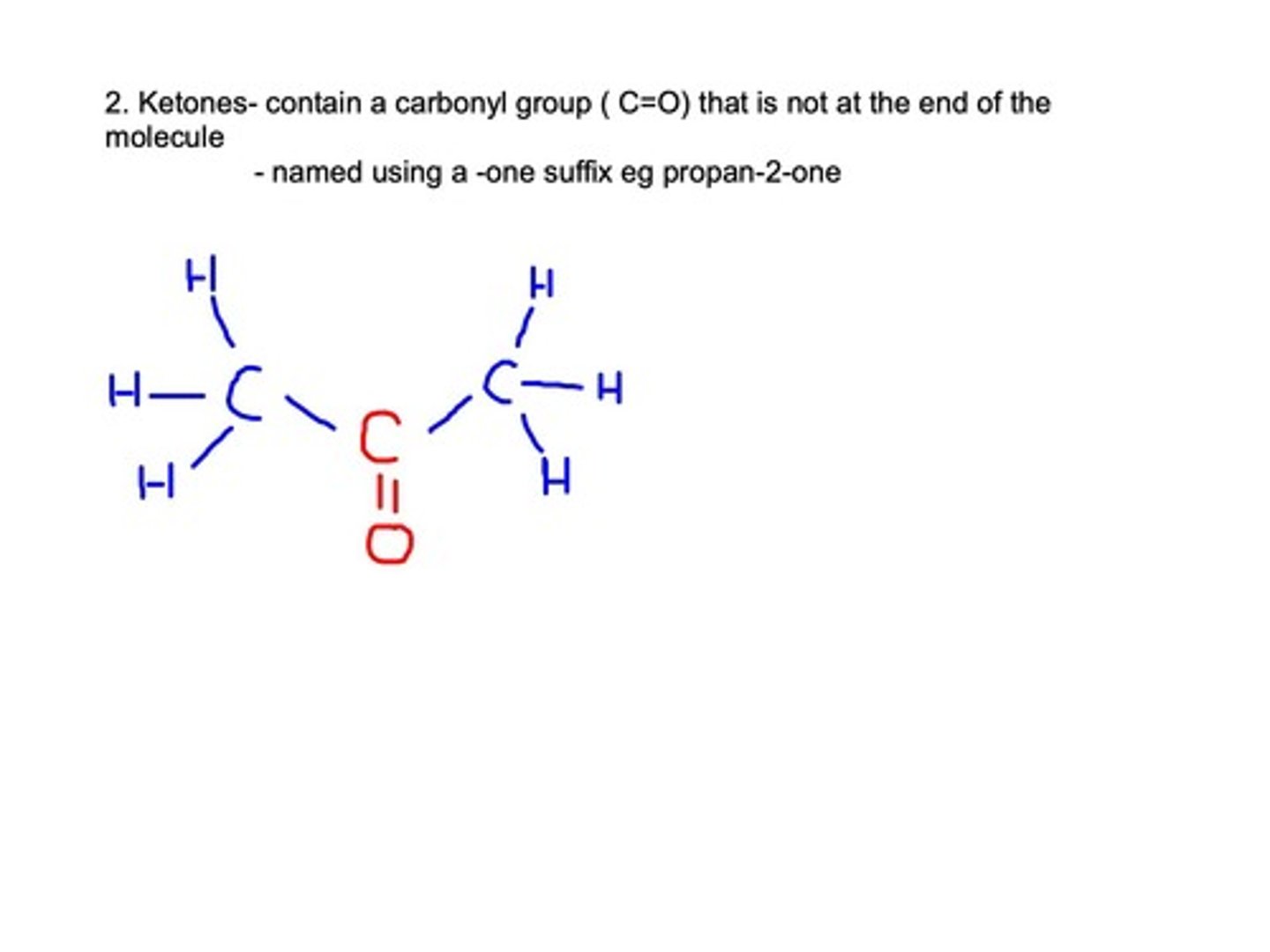
Ketones
Contain carbonyl (CO) group not at the end of the molecule.
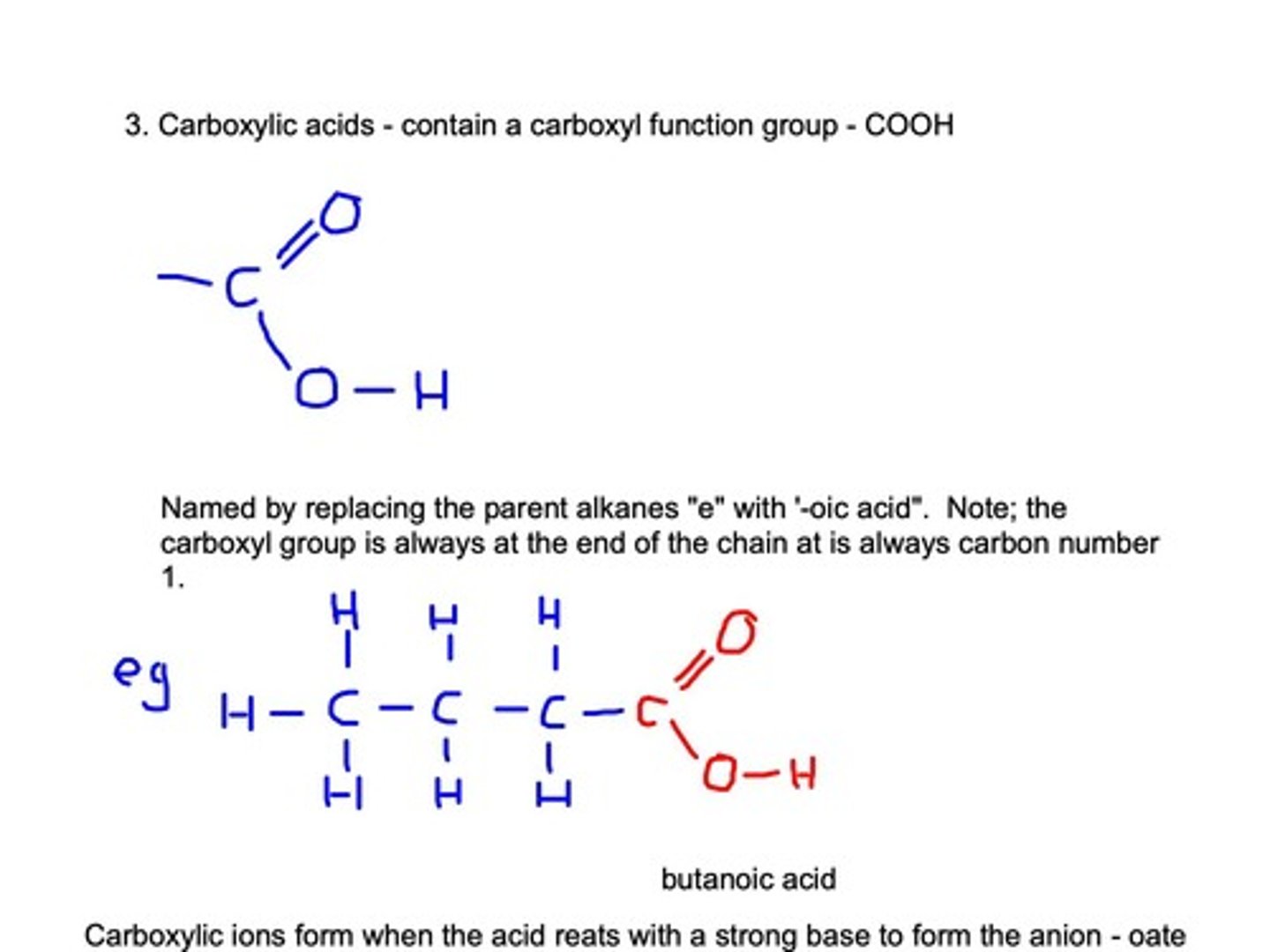
Carboxylic Acids
Contain carboxyl group (-COOH) at the end.
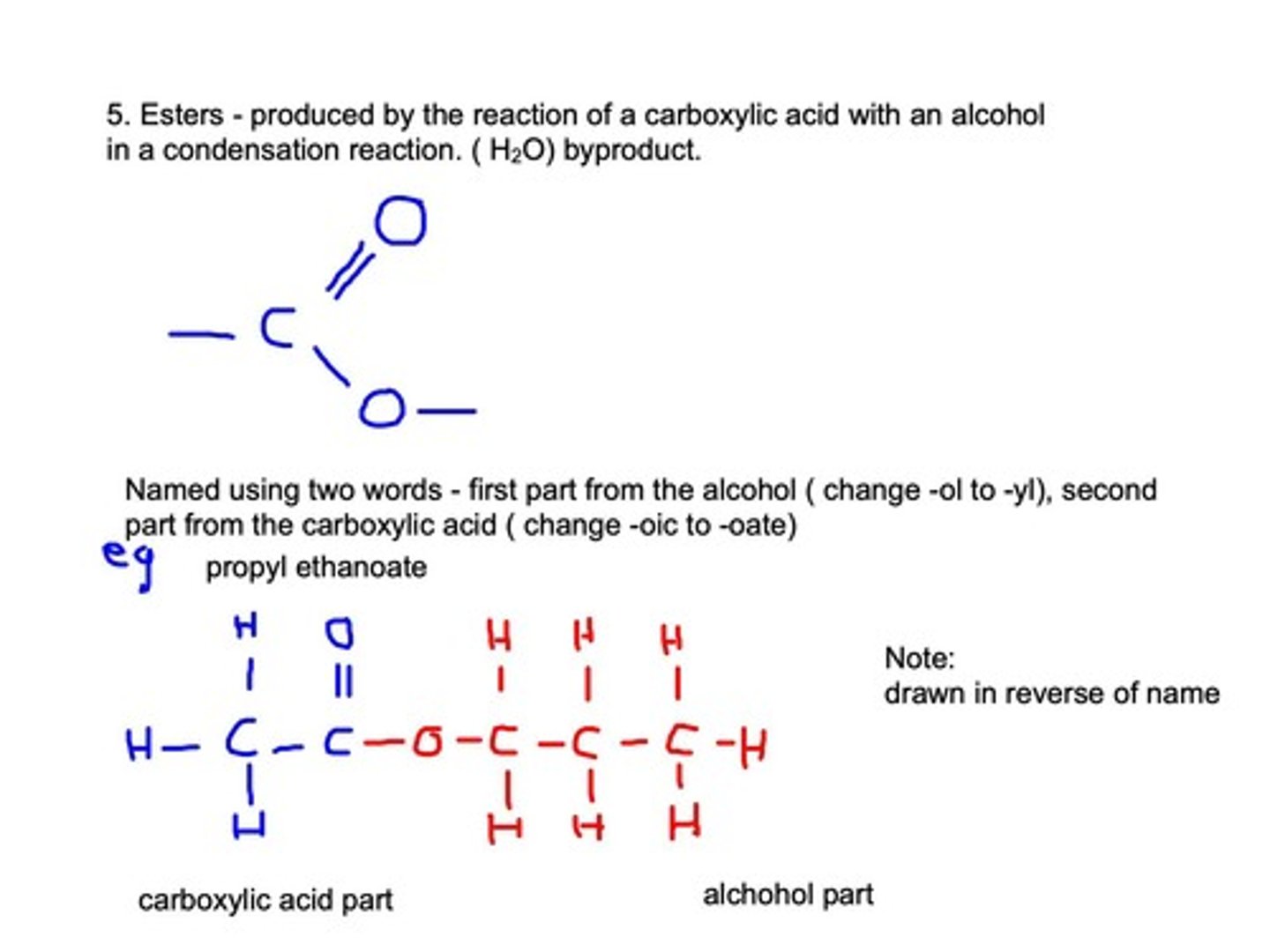
Esters
Formed from reaction of alcohol and carboxylic acid.
Primary Amines
Contain amino group (-NH2) attached to carbon.
Condensation Reaction
Reaction that produces water as a byproduct.
Hydrolysis
Splitting molecules using water, often enzyme-catalyzed.
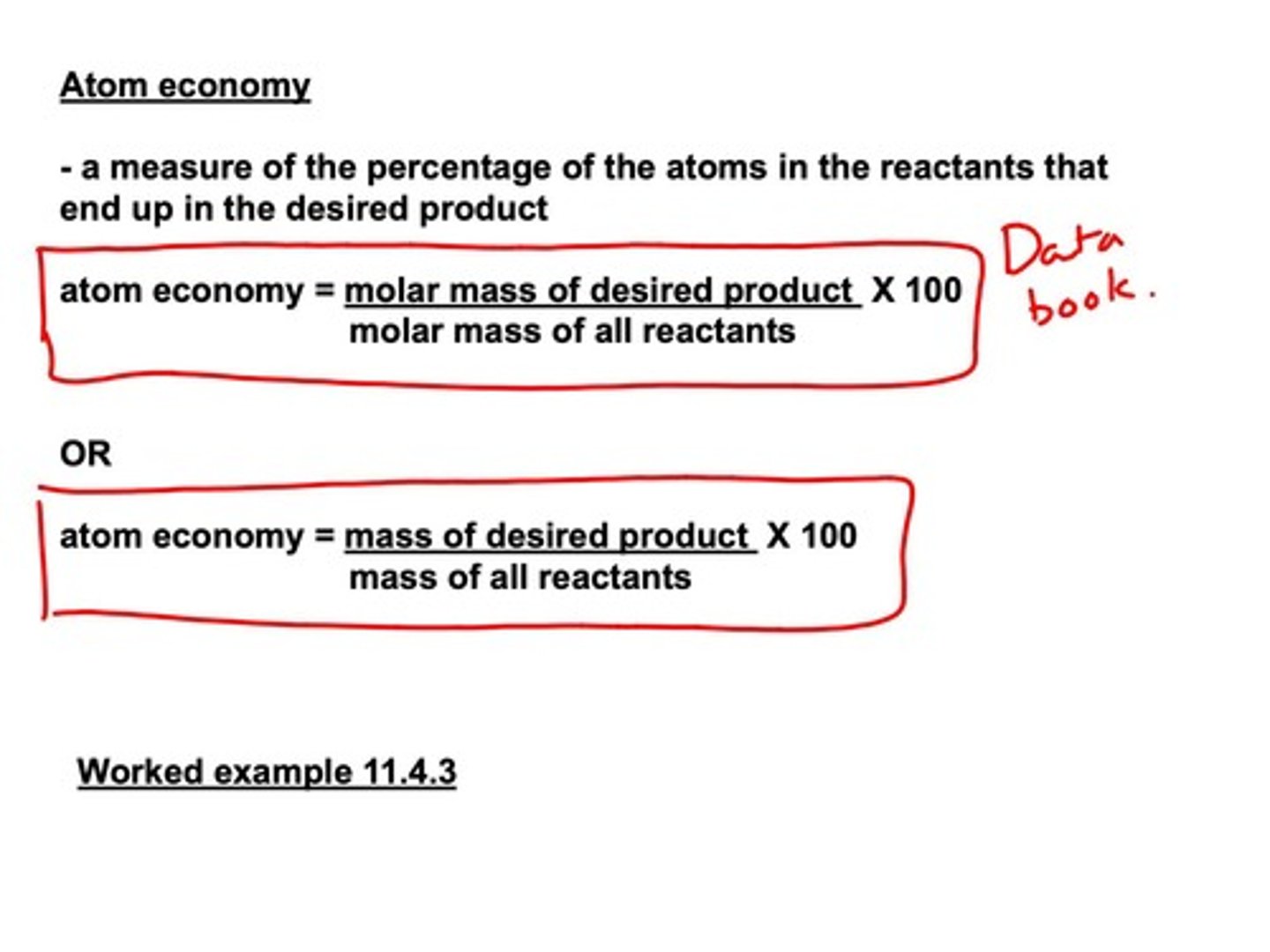
Atom Economy
Percentage of reactant atoms in desired product.
Percentage Yield
(Actual yield/Theoretical yield) x 100.

Intermolecular Forces
Forces between molecules affecting physical properties.
Dispersion Forces
Weakest intermolecular forces present in all compounds.
Dipole-Dipole Forces
Attractive forces between polar molecules.
Hydrogen Bonds
Strong bonds between H and O, N, or F.
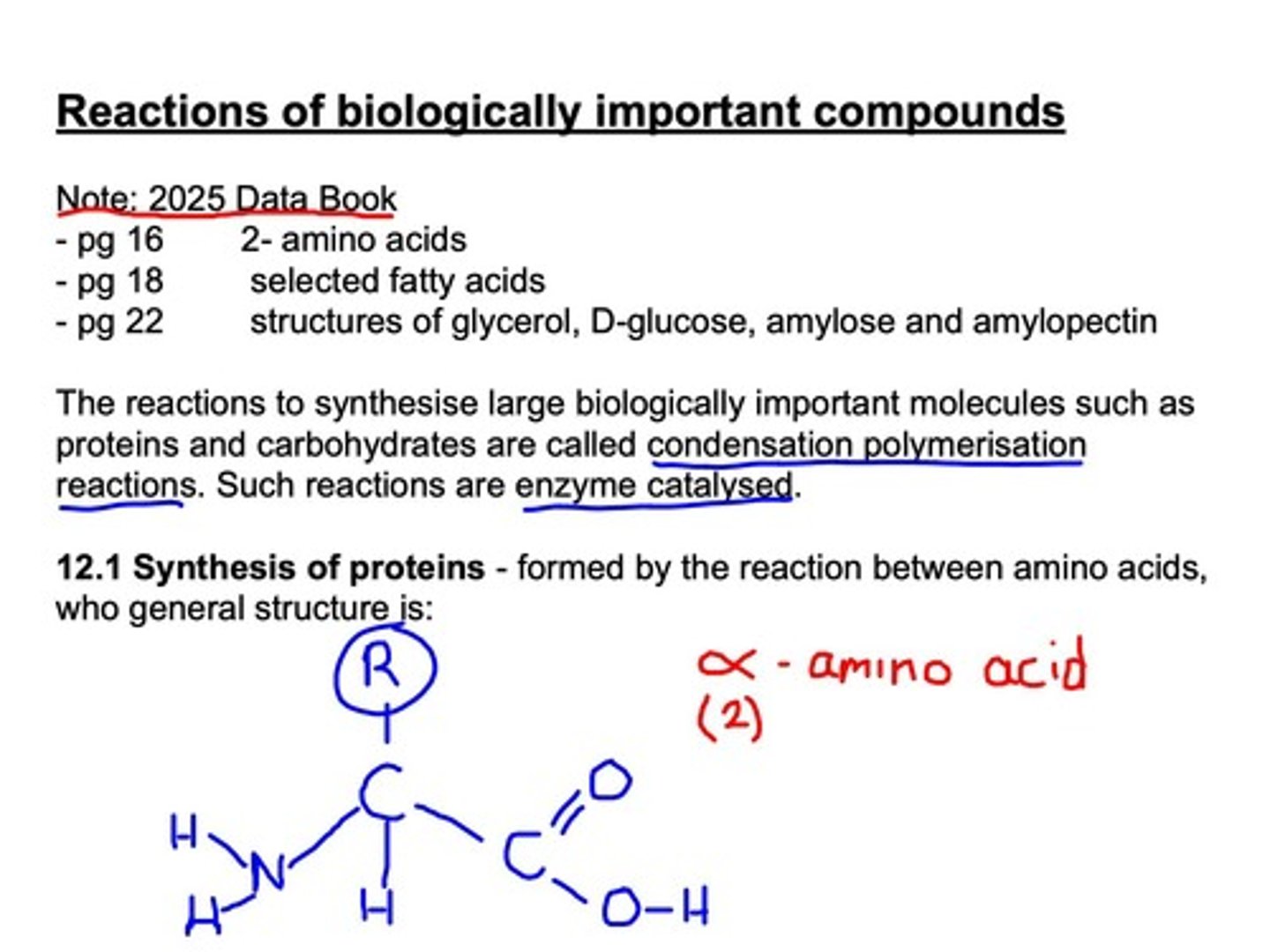
Polypeptides
Chains of amino acids linked by peptide bonds.
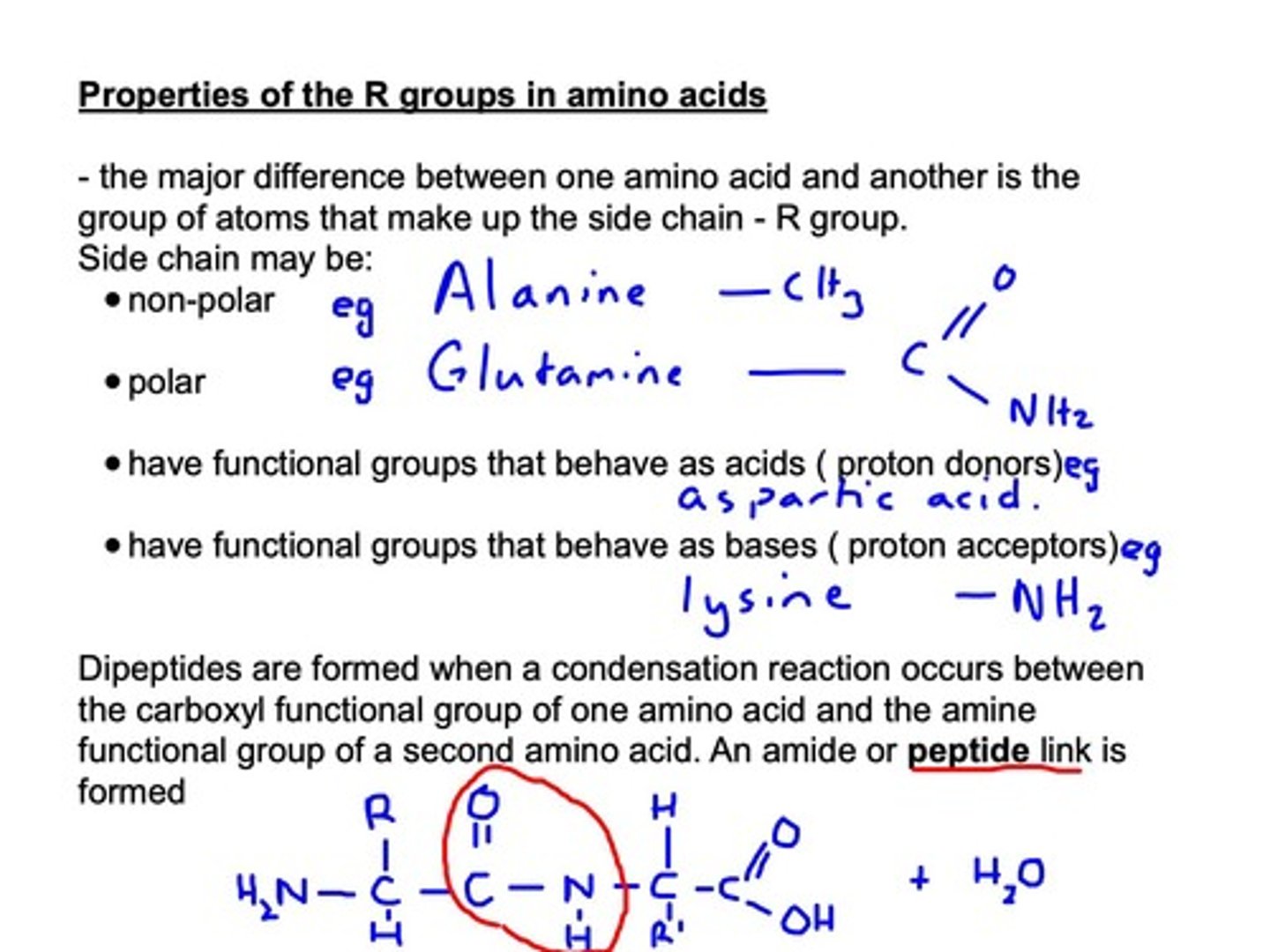
Dipeptides
Formed from two amino acids via condensation.
Monosaccharides definition
Simplest carbohydrates, e.g., glucose, fructose.

Disaccharides definition
Two monosaccharides linked by glycosidic bond.
Polysaccharides definition
Long chains of monosaccharides, e.g., starch, cellulose.
Saturated Fatty Acids
Only single carbon-carbon bonds in hydrocarbon chain.
Unsaturated Fatty Acids
Contain one or more double bonds in hydrocarbon chain.

Boiling point
temperature at which a substance changes from a liquid to a
gas at a specific pressure.
how does Molecular Size influence boiling point
makes a higher boiling points because they have a greater number of electrons, leading to stronger van der Waals forces between molecules
how does molecular mass influence boiling point
Heavier molecules with greater mass typically have
higher boiling points due to the increased number of electrons and stronger London dispersion forces.
how does hydrogen bonding influence boiling point
Molecules capable of forming hydrogen bonds,
typically those with -OH, -NH2, or -COOH groups, have significantly higher boiling points because of the strong hydrogen bonds between molecules
how does polarity influence boiling point
Polar molecules with permanent dipoles, such as those containing electronegative atoms, have higher boiling points
due to stronger dipole-dipole interactions
viscosity
the resistance of a liquid to flow
how does molecular size and shape influence viscosity
Larger and more complex molecules tend to
have higher viscosities because they have more extensive molecular interactions. Highly branched or irregularly shaped molecules may have
lower viscosities.
how does polarity influence viscosity
Polar molecules exhibit higher viscosities compared to non polar molecules of similar size due to stronger intermolecular forces.
how does temperature influence viscosity
Viscosity generally decreases with increasing temperature because higher temperatures provide molecules with more kinetic energy to
overcome intermolecular forces, making the liquid flow more easily.
flashpoint
the lowest temperature at which a substance's vapour can ignite when exposed to an open flame or spark.
how does volatility influence flashpoint
Highly volatile compounds with low boiling points tend to have lower flashpoints because they can vaporise easily.
how does chemical structure influence flashpoint
Some functional groups, such as alkyl groups (-CH3), can lower flashpoints because they promote vaporisation. Conversely, polar
or functional groups may increase flashpoints.
how does ignition sources influence flashpoint
The presence of ignition sources, such as open flames or sparks, can lower the effective flashpoint of a substance by providing the
necessary energy for ignition.
esterfication reaction
carboxylic acid + alcohol --> ester + water
addition reaction
a chemical reaction in which two or more substances combine to form a new compound (c=c)
substitution reaction
a reaction in which one or more atoms replace another atom or group of atoms in a molecule (c-c)
hydrolysis reaction
water is used to break down a polymer (protein)
condensation reaction
monomers join to form polymers (proteins) and water molecules are also formed
oxidation reaction
a reactant loses one or more electrons, thus becoming more positive in charge (Cr2O7 or MnO4)
examples of monosaccharides
glucose, fructose galactose (C612O6)
Disaccharides
carbohydrates formed from the reaction of two monosaccharides via a condensation reaction between the hydroxyl groups forming an ether
Polysaccharides
generally insoluble in water and have no taste
Triglycerides
made from a condensation reaction between a glycerol molecule and three fatty acids
Polyunsaturated fatty acids
a type of fat characterized by having two or more carbon-carbon double bonds in their chemical structure
how does chain length influence solubility
As the length of the hydrocarbon chain increases, the non-polar hydrocarbon part of the molecule starts to become more important and the solubility decreases.
how does pressure affect solubility
As you increase the pressure of a gas, the collision frequency increases and thus the solubility goes up, as you decrease the pressure, the solubility goes down
how does temperature affect solubility
According to Le Chatelier's Principle, the system adjusts to this increase in the heat by promoting the dissolution reaction to absorb the added heat energy. Increasing the temperature, therefore, increases the solubility of the solute.
how to test for a carbon to carbon double bond
Add bromine or iodine. The double bond is broken and there is an addition reaction
how to test for carboxylic acids
litmus paper, add alcohol, add a base, universal indicator
general formula for making esters
RCOOH (aq) + ROH (l) -> RCOOR(l) + H2O(l)