Chapter 5 (Chemistry: The Central Science)
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
64 Terms
Define Kinetic Energy
and equation
energy of motion
v = velocity
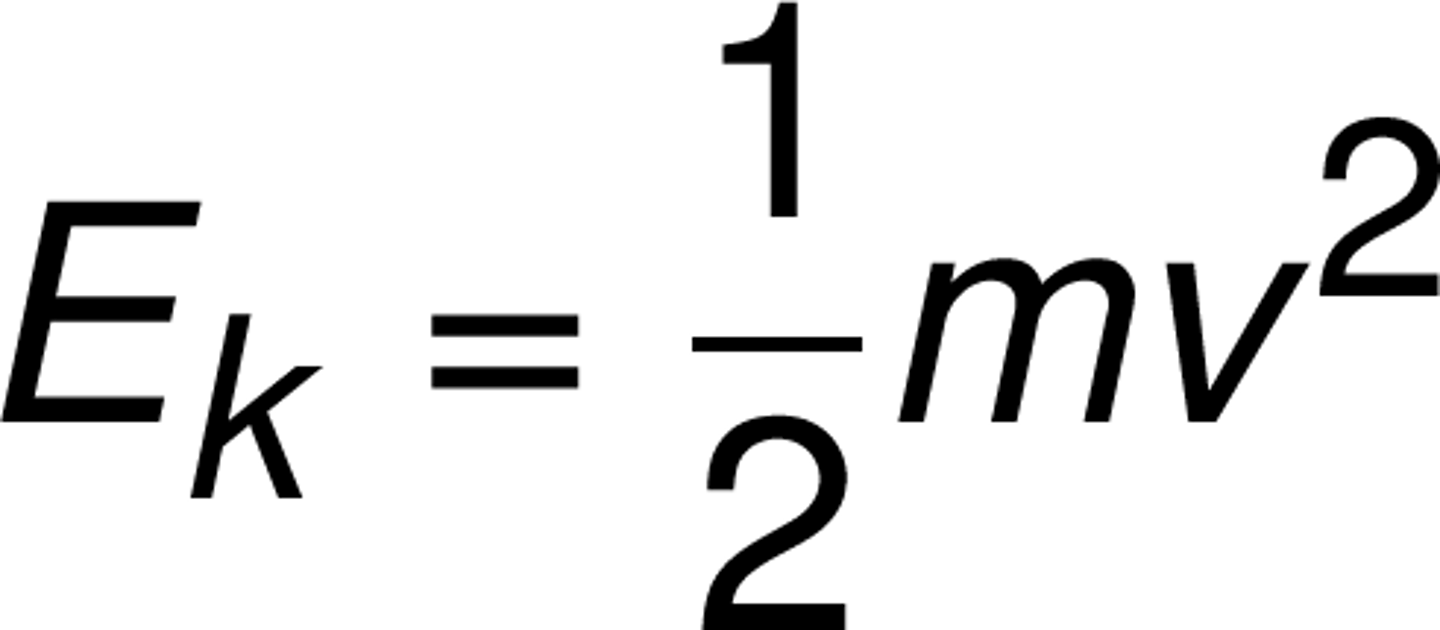
Joule unit
what are joules a measure of?
energy
work
heat by extension

conversion of 1 liter of atmospheric pressure to Joules
1 L-atm = 101.3J
Calorie -joule conversion
4.184J
define internal energy
what is it's sign
What is it's formula
sum of all kinetic/potential energies of the components of a system
denoted by E
Final value - initial value = E
What does ΔE stand for
change in internal energy
How to calculate change in internal energy of a system
change in internal energy
ΔE = q + w
q = heat (quantity of)
q and w can be positive or negative depending on if work/heat is being absorbed/done on the system or released/done by the system
two conditions that effect internal energy
temperature and pressure
State Function -
Does E depend on the path a system takes to get to it's final state (all those energy changes)?
NO, only the current state
You could heat a pot of water to 25 degrees C or cooling to 25 degrees C. Both are still 25 degrees C
Since E is a state function what does ΔE depend on?
initial and final states, not how the change occurs (heating vs cooling)
Enthalpy definition
H = E + PV
a thermodynamic quantity equivalent to the total heat content of a system. (potential of a system to create heat)
Change in enthaply
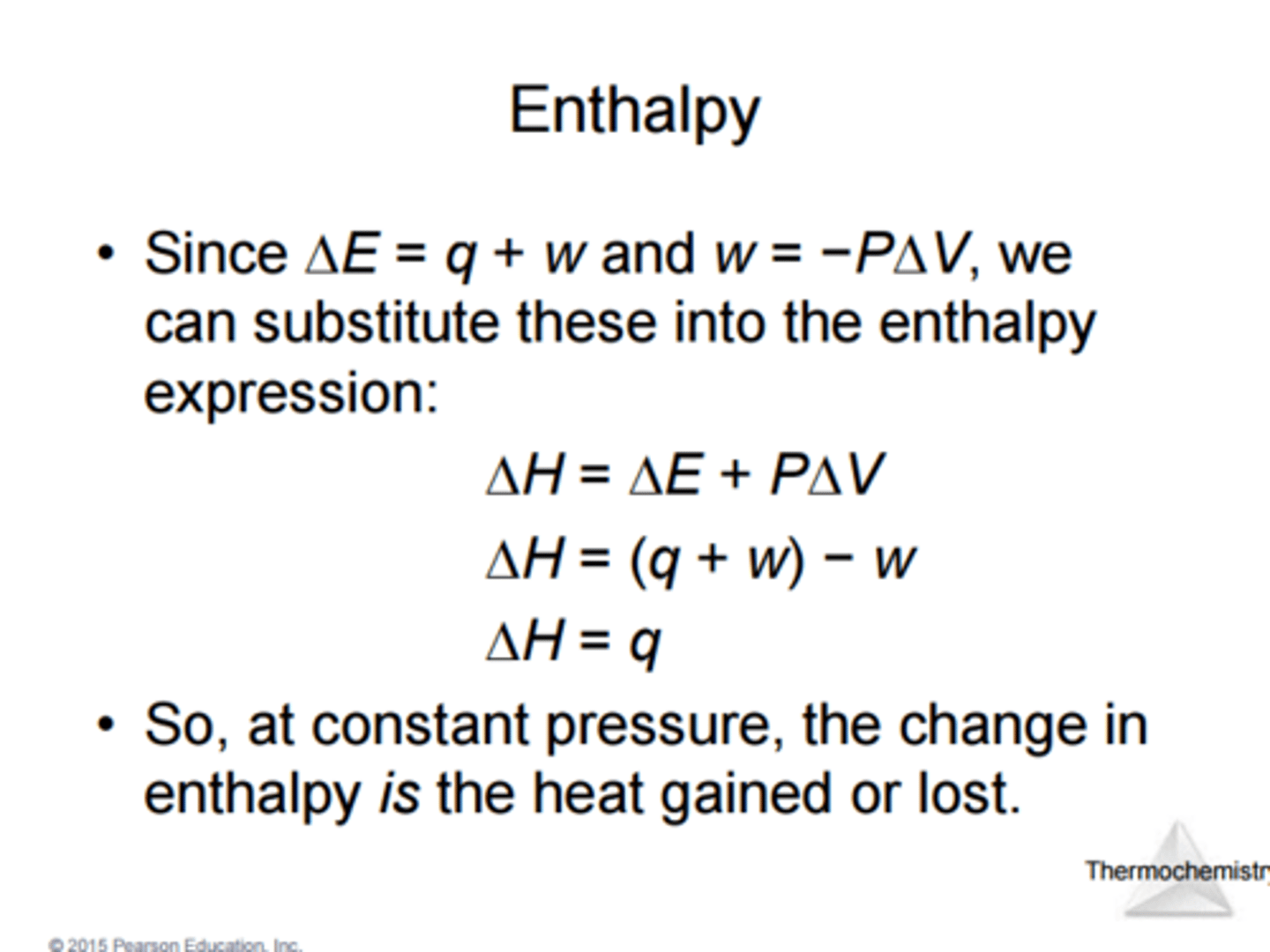
Pressure-volume work equation
w = -PΔV
ΔV = Vfinal - Vinitial
conversion of 1 liter of atmospheric pressure to Joules
1 L-atm = 101.3J
Define specific heat
Specific heat sign
Specific heat of water
Heat reqired to reaise temp of one gram of substance 1K (1 degree Celsius). It's a measure of heat capacity
Cs
water-
1.00cal/g(Celsius) OR 4.18J/g(Celsius)
Specific heat equation
Heat transferred/mass x temperature change
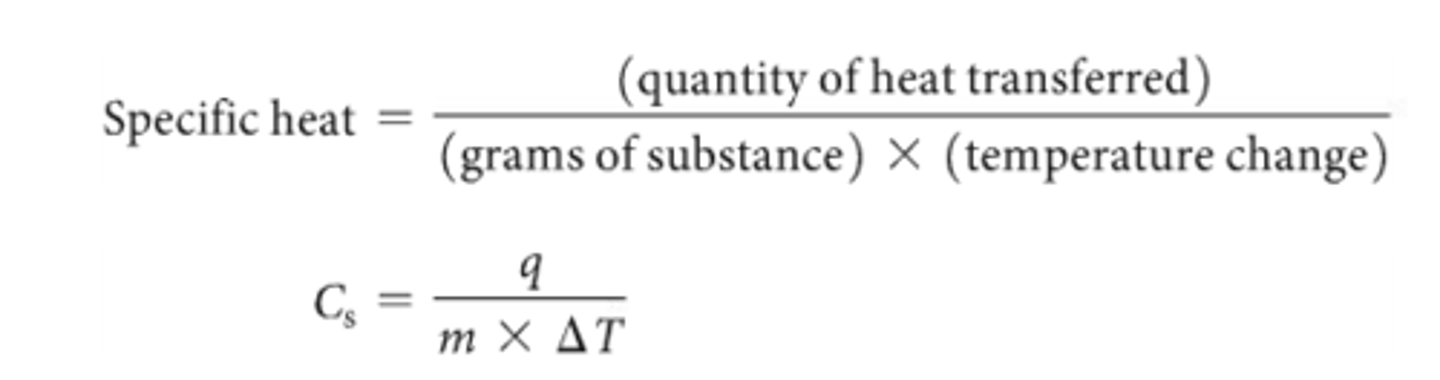
what is q
a quantity of temperature
Constant pressure caloimetry equation
q(soln) = m x Cs x ΔT = -q(rxn)
So, q(rxn) = -m x Cs x ΔT
reactants and products have same number but opposite sign
When doing caloemetry...
Assume perfect calometer
Assume density and specific heat of solution is same as water
specific heat of water is 4.18 J/g-K
What does ΔH depend on
initial state, final state, amount of matter involved
Enthaply of formation, ΔHf
aka; Heat of formation)
the enthaply change when ONE MOLE of the product is formed from it's elements in their standard states
The enthalpy of formation of any element in it's standard state is zero
(list of standard enthalpies of formation in back of book)

equation for using Hess's law and Enthalpy of formation to determine (ΔH
Remember ΔHf is Enthalpy of formation
n and m = the coefficients
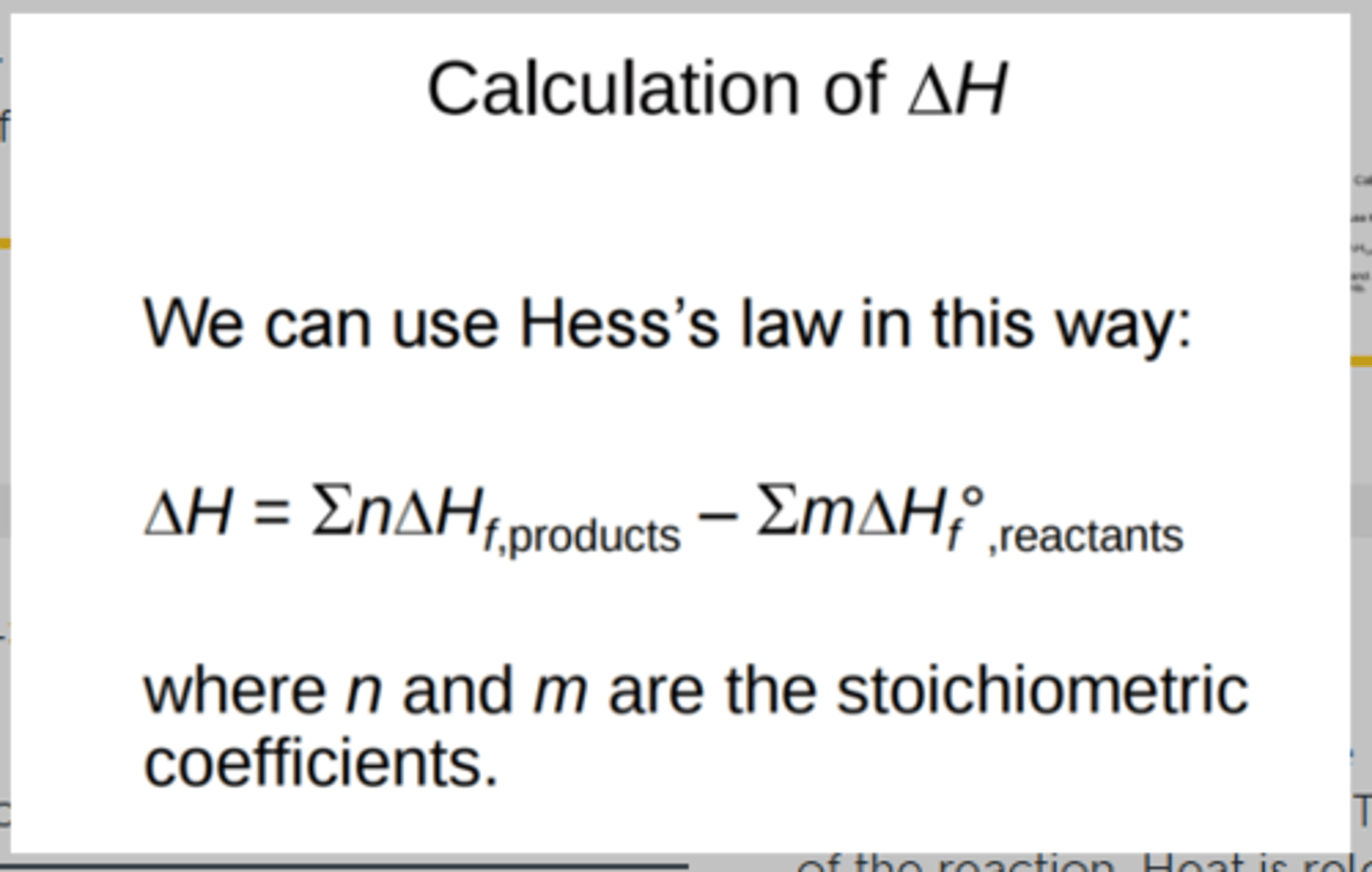
When canceling out parts of equations do the coefficents have to be the same? YES and you can cancel only some of the coefficient I think
thermodynamics
study of energy and its transformations
(section 5.0)
thermochemistry
study of relationships between chemical reactions and energy changes
subset of thermodynamics
(section 5.0)
energy
(E)
capacity to do work or transfer heat (unit: J)
(section 5.1)
work
(w)
energy used to cause an object to move against a force (unit: J)
w = F×d = m×a×d
∙ +/- (vector quantity)
∙ F (force, N)
∙ d (distance, m)
∙ m (mass, kg)
∙ a (acceleration, m/s2)
(section 5.1)
heat
(q)
energy used to cause the temperature of an object to increase (unit: J)
-A form of energy measured in Joules (J)
(section 5.1)
kinetic energy
E(k)
energy of motion (unit: J)
E(k) = ½ m v²
∙ m (mass, kg)
∙ v (velocity, m/s)
(section 5.1)
potential energy
E(p)
energy an object has by virtue of its position relative to other objects
stored energy that arises from attraction or repulsion an object experiences in relation to other objects
E(p) = m g h = F d
∙ m (mass, kg)
∙ a (acceleration, m/s²)
∙ d (distance, m)
∙ F (force, N)
(section 5.1)
electrostatic potential energy
E(el)
potential energy that arises from interaction between charged ions
E(el) = (k Q₁ Q₂) / d
∙ k (constant, J-m/C²)
∙ Q₁,Q₂ (charges of interacting objects, C)
∙ d (distance, m)
(section 5.1)
thermal energy
the energy a substance possesses because of its temperature.
joule
(J)
SI unit for energy
1 J = 1 kg-m²/s²
kinetic energy of a 2 kg object moving at 1 m/s:
E(k) = ½ × 2 kg × (1 m/s)² = 1 kg-m²/s² = 1 J
(section 5.1)
calorie
(cal)
1 cal = 4.184 J (exactly)
originally, amount of energy required to raise temperature of 1 g of water from 14.5°C to 15.5°C
(section 5.1)
system
matter being considered
may be OPEN, CLOSED, or ISOLATED
(section 5.1)
open system
system in which matter and energy may be exchanged with surroundings
examples:
∙ uncovered pot of boiling water
∙ reactants in aquieous solution inside of a calorimeter
(section 5.1)
surroundings
space and matter outside of the system
(section 5.1)
closed system
system in which energy may be exchanged with surroundings, but not matter
example
∙ mixture of H₂ and O₂ inside of engine piston
(section 5.1)
isolated system
system in which neither energy nor matter may be exchanged with surroundings
example
∙ thermostat (approximates isolated system, though not perfectly)
(section 5.1)
work
Energy transferred when a force moves an object
w= F x d (Work=force x distance)
force
(F)
push or pull exerted on an object
F = m×a
∙ m (mass, kg)
∙ a (acceleration, m/s²)
(section 5.1)
Heat
the energy transferred from a hotter object to a colder one
first law of thermodynamics
assumption that energy is conserved in any process; it is neither created nor destroyed.
∆E = q + w
(section 5.2)
internal energy
(E)
sum of all kinetic and potential energies of the components of the system
(section 5.2)
change in internal energy
(∆E)
∆E = E(final) - E(initial)
∙ +/- (b/c vector quantity)
∙ state function b/c depends only on initial and final states of system, not path of change
∙ at constant volume, equals change in heat lost or gained (q)
(section 5.2)
endothermic
reaction
reaction causing system to gain (absorbs) heat
example
∙ melting of ice
(section 5.2)
exothermic
reaction
reaction causing system to lose (expand) heat
example
∙ combustion
(section 5.2)
state function
property of a system that is determined by specifying the system condition, or state (such as pressure or temperature)
value of state function depends only on present state of system, not the path the system took to reach that state
(section 5.2)
enthalpy
(H)
thermodynamic quantity equivalent to the total heat content of a system. It is equal to the internal energy of the system plus the product of pressure and volume
H = E + P×V
∙ E (internal energy of system)
∙ P (pressure of system)
∙ V (volume of system)
∙ enthalpy is an example of a "state function"
(section 5.3)
pressure-volume work
(P-V work)
w = -P×∆V
∙ - (b/c system does work on surroundings)
∙ P (pressure; no ∆ b/c constant)
∙ ∆V (change in volume)
(section 5.3)
change in enthalpy
(∆H)
∆H = ∆E + P×∆V = q(p)
∙ ∆E (change in internal energy)
∙ P (pressure of system)
∙ ∆V (change in system volume)
∙ q(p) (heat gained/lost by system; subscript "p" indicates process under constant pressure)
∙ at constant P, equals heat gained/lost (q)
(section 5.3)
enthalpy of reaction
(∆Hrxn)
enthalpy change associated with a reaction
aka. "heat of reaction"
(section 5.4)

thermochemical equation
balanced chemical equation that shows associated enthalpy change
example
∙ 2 H₂(g) + O₂(g) → 2 H₂O(g) ∆H = -483.6 J
properties of enthalpy
∙ extensive property (magnitude proportional to amount of reactants consumed in process)
∙ equal in magnitude, opposite in sign for reverse reactions
∙ depends on physical state of reactants and products (gas, liquid, solid)
calorimetry
area of chemistry dealing with measurement of heat flow
(section 5.5)
calorimeter
device used to measure heat flow
types include
∙ coffee-cup
∙ bomb
(section 5.5)
heat capacity
temperature change experienced by an object of a certain mass (i.e. grams, moles) when it absorbs a certain amount of heat
(section 5.5)
molar heat capacity
(Cm)
heat capacity of 1 mole of substance (
C(s) = q / (m (mol/amu))×∆T
∙ q (qty H transferred, J)
∙ m (mass, g)
∙ mol (1 mole)
∙ amu (atomic mass of compound in grams)
∙ ∆T (change in temperature, °C or K)
(section 5.5)
specific heat capacity
(Cs)
heat capacity of 1 gram of substance
C(m) = q / m×∆T
∙ q (qty H transferred, J)
∙ m (mass, g)
∙ ∆T (change in temperature, °C or K)
unit
∙ kJ/g-K(°C) for "bomb" calorimeters
∙ J/g-K(°C) for "coffee-cup" calorimeters
(section 5.5)
coffee-cup calorimeter
a simple calorimetric device made of two nested coffee cups. Device isn't sealed and is used in "constant-pressure" calorimetry
heat capacity
∙ C(s) (specific heat, J/g-K)
(section 5.5)
bomb calorimeter
device for measuring the
heat evolved in the combustion of a substance under constant-volume conditions
q(rxn) = -C(cal)×∆T = -q(soln)
heat capacity
∙ C(cal) (total heat capacity of calorimeter, kJ/K or °C)
(section 5.5)
Hess's Law
If a reaction is carried out in a series of steps, ∆H for the overall reaction equals the sum of enthalpy changes for individual steps
(section 5.6)
enthalpy of formation
∆H that accompanies the formation of a substance from the most stable forms of its component elements
∆H based on addition together of thermochemical data of constituent elements
aka. "heat of formation"
(section 5.7)
standard enthalpy of formation
(∆H°f)
∆H for the reaction that forms 1 mole of compound from its elements with all substances in standard states
(section 5.7)