MAE 3324 Final Exam
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
71 Terms
Tensile vs Yield Strength
Tensile is the maximum stress a material can withstand while being stretched before breaking; Yield is the stress at which a material begins to deform plastically
Hardness
The resistance of a material to localized plastic deformation, such as indentation or scratching (Rockwell Test)
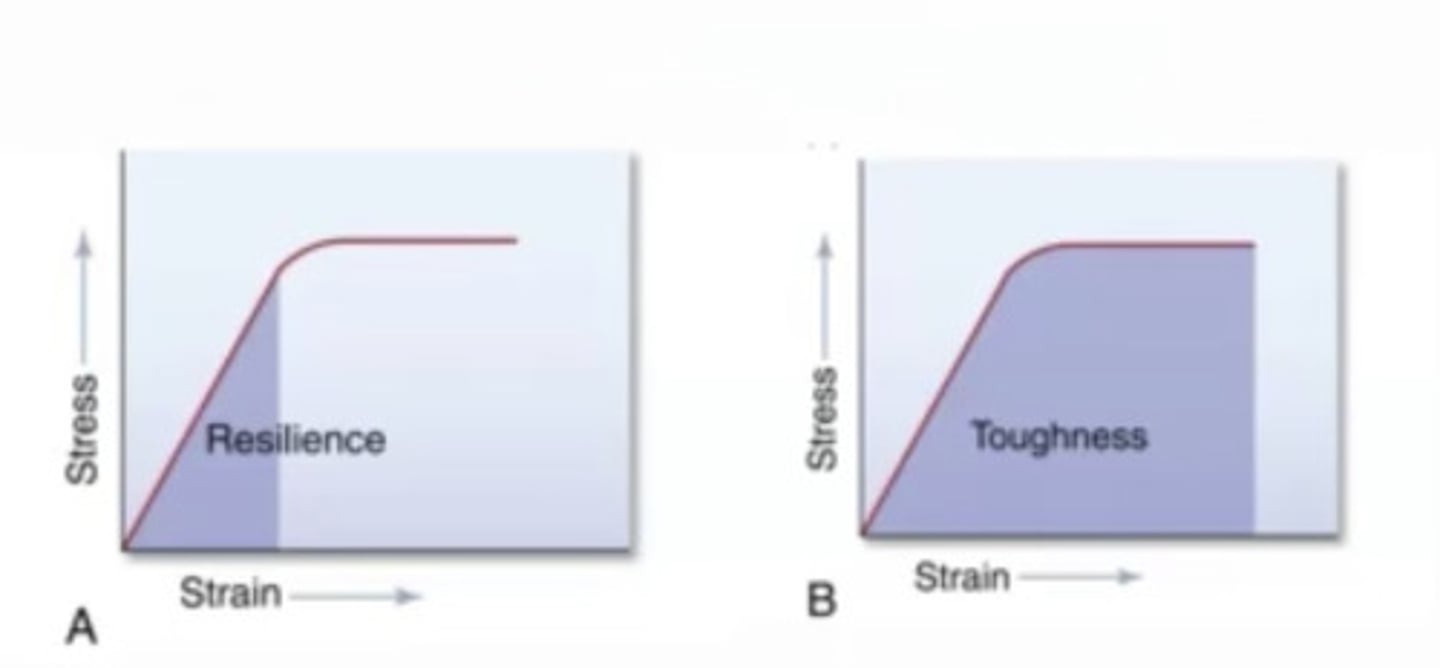
Toughness
The ability of a material to absorb energy and deform plastically without fracturing
Stress-Strain Curve
A graph showing the relationship between the applied stress and the resulting strain, Elastic Modulus, Yield Strength, and ultimate tensile strength
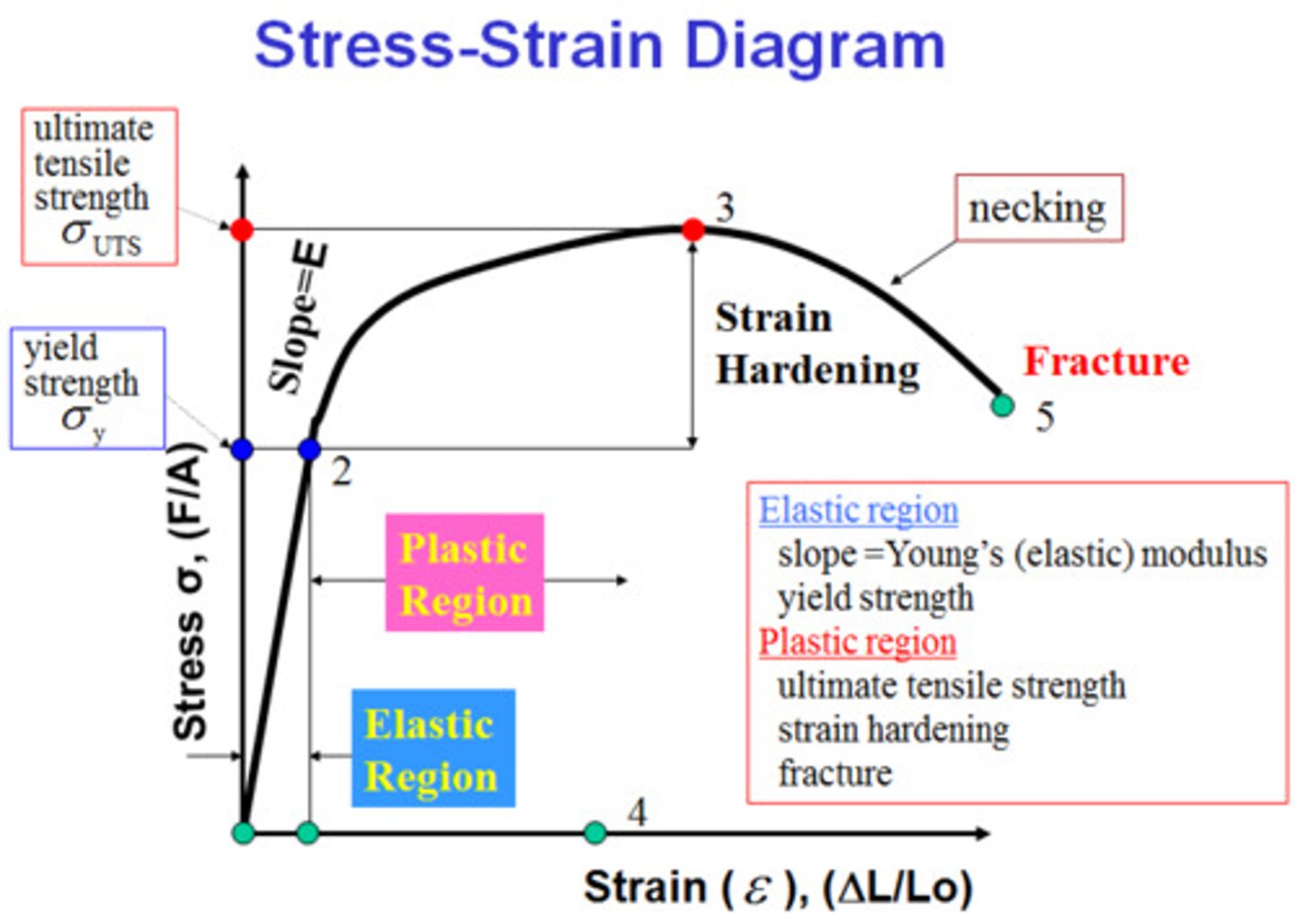
Elastic Modulus
The ratio of stress to strain in the elastic region, representing stiffness
Engineering vs True Stress
True stress considers the changing cross-sectional area while engineering just considers the original
Strengthening
The process of improving the mechanical properties of a material, alloying, work hardening, or heat treatment
Cold Work (Strain Hardening)
A metalworking process where the material is plastically deformed at or near room temperature, increasing its strength and hardness
Grain Size Reduction
A method of strengthening a material by decreasing the size of its grains, smaller grains act as barriers to dislocation movement
Solute Strengthening
Adding alloying elements to form solid solutions, disrupting the regular lattice structure making it more difficult for dislocations to move
Phase Transformations
Changes in the microstructure of a material that alter its properties (Ex: Austenite to Martensite)
Precipitation
The formation of finely dispersed particles (precipitates) within a metal matrix from a supersaturated solid solution, which can enhance the material's strength and hardness by impeding dislocation movement
mepbp
Methane CH4
Ethane C2H6
Propane C3H8
Butane C4H10
Pentane C5H12
ECFPS
Polyethylene (PE C2H4)
Poly(Vinyl Chloride) (PVC C2H3Cl)
Polytetrafluoroethlyne (PTFE C2F4)
Polypropylene (PP C2H3(CH3))
Polystyrene (PS C2H3(C6H3))
Creep
Time-dependent deformation of a material under a constant load
Glass transistion
The reversible change in a material from a hard, glass state to a soft rubbery state as it is heated
Fiber in Martix
Fibers strengthen a matrix by transferring load to themselves through their high tensile strength
3 Steps in Precipitation
1. Solution Treatment (Heating to dissolve alloying elements into a solid solution)
2. Quenching (Rapid Cooling to retain solute atoms in the matrix)
3. Aging (Formation of fine precipitates to strengthen the material
Dislocation Hardening
Dislocations move through the material under stress; strengthening occurs by impeding the movement of these dislocations (Grain Size Reduction, Work Hardening, Solution Strengthening, Precipitation Hardening)
Overaging
The fine precipitates coarsen and become less effective at obstructing dislocations because the size increases, reducing the strength imparted to the material
Fatigue Limit (Endurance Limit)
The maximum stress level a material can endure for an infinite number of cycles without experiencing fatigue failure
Fatigue Strength
The maximum strength a material can handle for a specified number of cycles before it fails
Atomic Packing Factor
APF = Volume of atoms in the unit cell/Volume of the Unit Cell
Coordination Number
The number of nearest neighboring atoms surrounding a particular atom in a crystal structure
Improving Fatigue Life
1. reducing magnitude of mean stress
2. surface treatments
3. design changes
Isomerism
2 Compounds with the same chemical formula, different physical structure (must break bonds to make them look the same)
Fatigue
Cracks that grow in the cyclic range
Grain Boundaries
Boundaries between like crystals, produced by solidification process
When will a crack propagate
When the energy stored in material as strain E exceeds that needed to create new fracture surfaces
Recrystallization
Eliminates defective grains
Grain Growth
After recrystallization is complete, the strain-free grains will continue to grow if the metal specimen is left at the elevated temperature
Driving force for Recrystallization
Reduction in strain energy
Hardenability
How easy is it for a material to form martensite
Two types of linear defects
Edge and Screw Dislocations
Two thermal properties of a liquid at medium that influence its quenching effectiveness
Thermal Conductivity & Heat Capacity
4 Measures that may be taken to increase the fatigue resistance of a metal alloy
1. Polish the surface to remove stress amplification sites
2. Reduce the number of internal defects by means of altering processing and fabrication techniques
3. Modify the design to eliminate notches and sudden contour changes
4. Harden the outer surface of the structure by case hardening or shot peening
Case Hardening
A treatment process used to harden the outer surface (Case) of a metal while keeping the inner core softer and more ductile
Shot Peening
A mechanical surface treatment process used to improve the fatigue life and mechanical properties of a material by inducing compressive residual stresses on its surface
Isomer
Compounds with the same formula but different structures.
Thermoplastic
Softens at higher temperatures, loosens the secondary bonds, allowing for the chains to slide past each other, linear or branched Molecular structure
Thermoset
Harden with temperature increase, chemical decomposition at extreme temperatures. Cross-linked or Networked Molecular structure
Crystallinity Polymers
Ordered atomic arrangements involving molecular chains, crystal structures in terms of unit cells
Copolymers
Two or more monomers polymerized together
Isotactic
R groups on same side of chain
Synodiotactic
R groups alternate sides
How does the Youngs modulus change after being cold worked
No change
Why are small-angle grain boundaries not as effective in interfering with the slip process as are high-angle grain boundaries
Low-misorientation boundaries with low energy and less disruption to atomic alignment, making them less effective at impeding dislocation motion compared to high-angle grain boundaries
Considering coarse pearlite, as C concentration increases why does ductility decrease?
Because we are loosing our ductile phase, Ferrite
When is there a driving force for nucleation
When below the melting temperature
When is surface free energy positive
Always
When is Gibbs Free Energy (ΔGv) negative?
When phase transformation is thermodynamically favorable (i.e Solidifcation)
Lattice Parameters
The dimensions and angles that define a unit cell in a crystal lattice, including lengths (a, b, c) and angles (α, β, γ), for BCC FCC etc calculate the lattice parameters (a) length using r, ex: for BCC a=4r/(3^.5)
Diffusion
The movement of atoms or molecules from high to low concentration due to thermal energy, driving material mixing over time
Steady State
A condition where the properties of a system remain consistent over time despite ongoing processes
Phase Diagram
A chart showing the stable phases of a material under different temperature and composition conditions
Eutectic
A specific composition in an alloy system where the lowest melting point occurs, forming two distinct solid phases simultaneously upon cooling
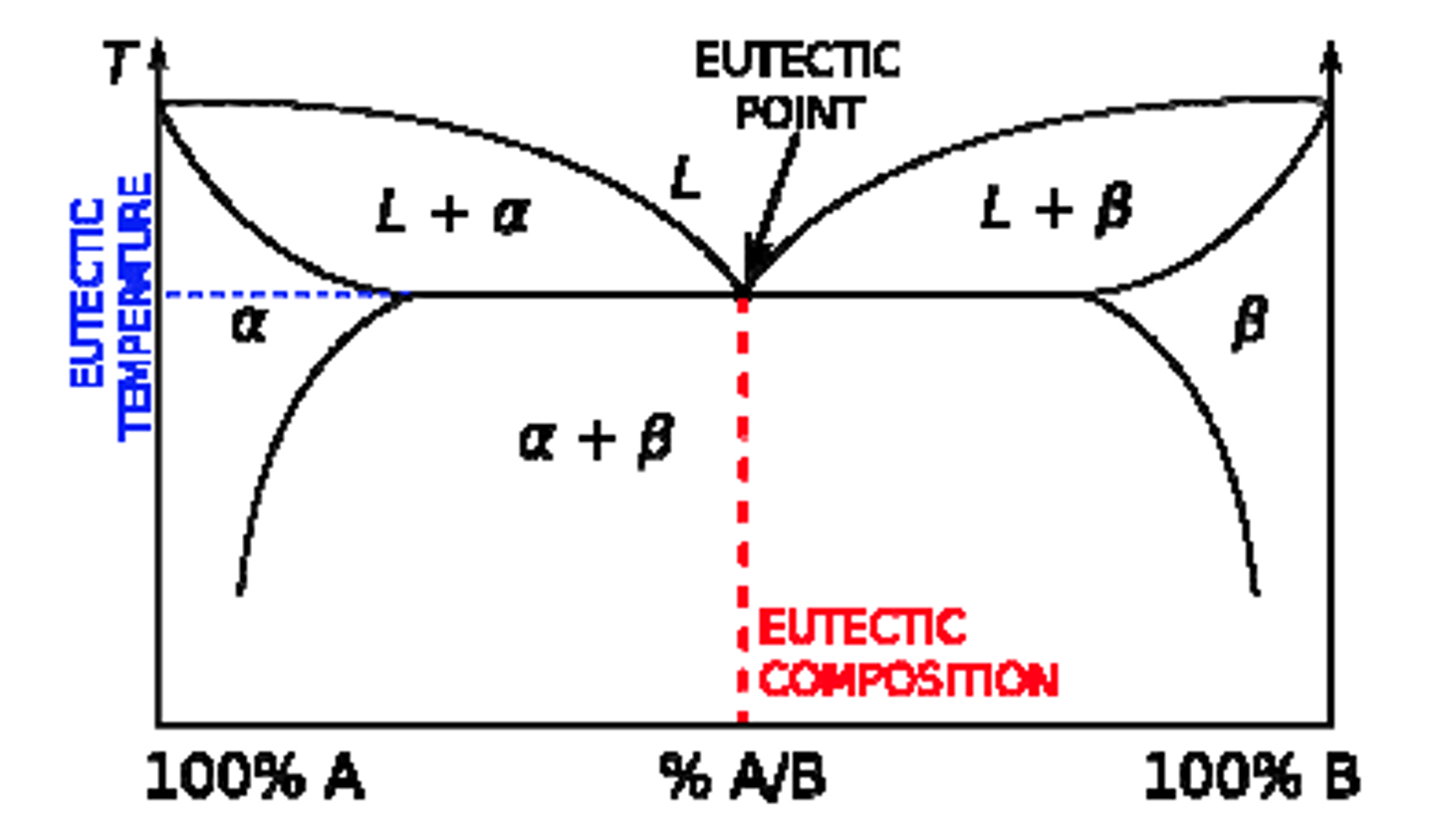
Eutectoid
A phase transformation where a single solid phase transforms into two different solid phases at a specific temperature and composition
Time-Temperature Phase Diagram
Shows how temperature and time affect phase transformations in materials, indicating phase stability and transformation rates
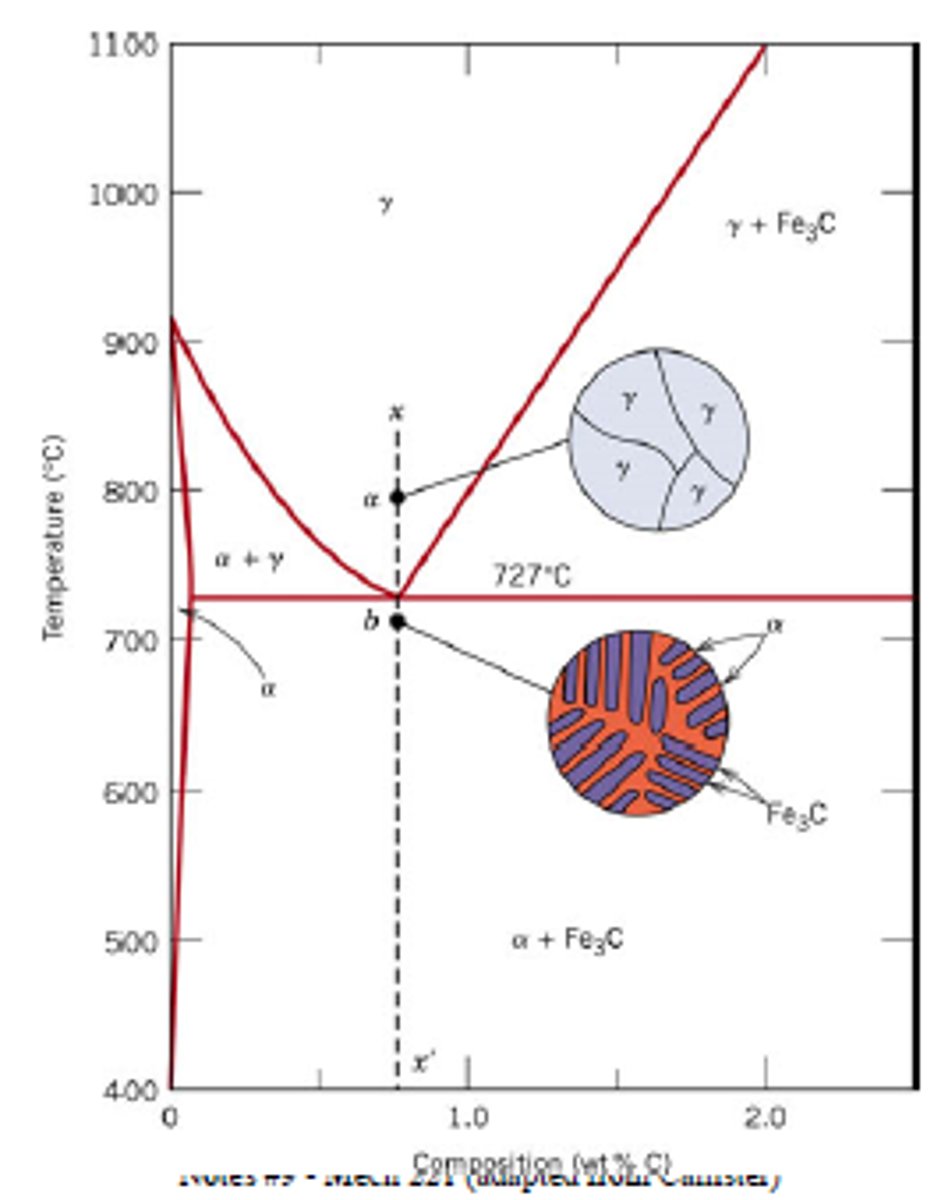
Iron Carbon Phase Diagram
Shows the phases and microstructures of iron-carbon alloys as a function of carbon content and temperature (ferrite, austenite, cementite)
Microconsituents
Distinct phases or structures in a material's microstructure (pearlite, bainite) that influence its mechanical properties
Microstructure
The arrangement of differnet phases, grains, and micro constituents within a material
Mechanical Properties
Various types of Mechanical forces, ductility, hardness, strength, & toughness
Ductility
The ability of a material to undergo significant plastic deformation before fracture, calculated using Area Reduction or Elongation %
Interchange
When atoms diffuse by swapping positions with their neighboring atoms, involves atomic movement that overcomes the energy barriers
Vacancy
When atoms diffuse into nearby vacant lattice sites
Intersticial
When small atoms (like hydrogen or carbon) diffuse through the interstitial spaces (gaps) between larger atoms in crystal lattice
Slip System
A crystallographic plane, with a direction along which dislocation motion (slip) occurs
Do all metals have the same slip system?
No, different metals have distinct slip systems depending on their crystal structure, affecting their ductility and mechanical properties
Recovery vs Recrystallization Process
Recovery relieves some internal strain energy by dislocation motion but virtually no change in grain structure or mechanical characteristics, Recrystallization forms a new set of strain-free grains
Driving force for recrystallization
∆Gv, change in internal energy
Driving force for grain growth
Reduction in grain boundary energy as the total grain boundary area decreases