MOLARITY AND MOLALITY
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Last updated 9:49 AM on 10/22/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
1
New cards
Concentration of Solutions
a measure of the amount of solute in a
given amount of solvent or solution
2
New cards
Percent by Mass
Percent by Volume
Percent by Mass-Volume
Mole fraction
Molality
Molarity
Parts per million
Ways to express concentrations of solution
3
New cards
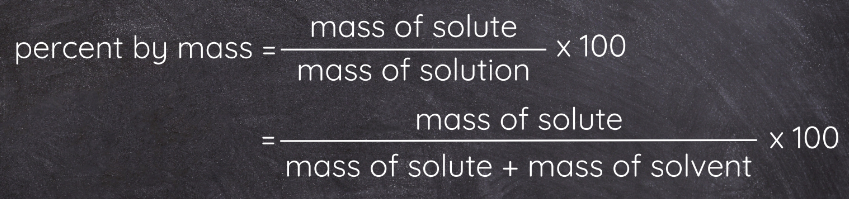
Percent by Mass (% w/w)
4
New cards
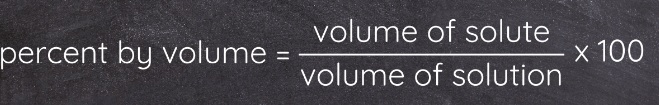
Percent by Volume (% v/v)
Involves the volume of both the solute and the solution.
5
New cards
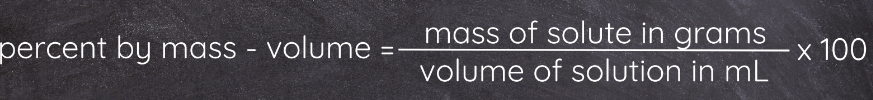
Percent by mass-volume (%w/v)
6
New cards
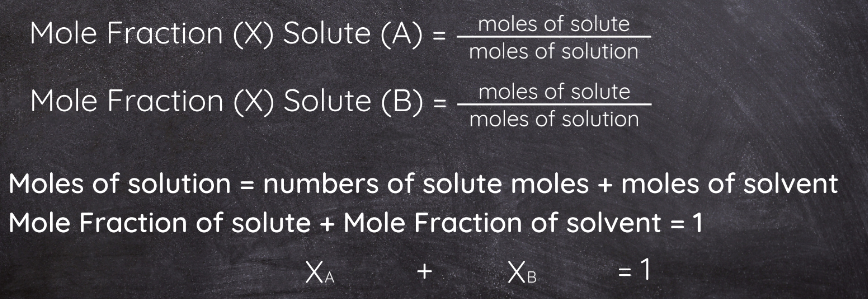
Mole Fraction
It is the ratio of the number of moles of one component to the total number of moles in a solution
7
New cards

Molality (m)
The number of moles of a solute per kilogram of solvent.