Chemical Principles Exam 1
1/34
Earn XP
Description and Tags
Modules 1-3. This set just contains Notes
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Exponents on a Calculator
-e or e^x
example: -2 × 10^5= 2e-5
Log on a calculator
may need to enter number first then Log.
example log(100)=2
Scientific Notation
cannot be greater than 10 nor less than one
Order of Operations
PEMDAS
Parantheses
Exponents
Multiplication
Division
Addition
Subtraction
Percentages
Percent (%) = /100
“of”= x (multiplication)
what= unknown quantity (x)
is = =(equals)
Slope
rise/run
y2-y1/x2-x1
chemistry
study of matter and energy
matter
anything that has mass and takes up space
elements
simplest form of matter
has distinct physical and chemical properties
building blocks of the universe
form attractions (Chemical bonds)
Chemical Bonds
formed by atoms of different elements
can be broken and form new bonds with different atoms
compound
chemical combination of elements that has its own set of properties and a chemical composition
pure water always contains the same two elements with the same proportions
88.8% oxygen, 11,2% hydrogen by mass
Can only be separated through chemical reactions
Chemical composition
the given ratio by mass of each element in a compound to any other element in the compound
Elements and Compounds are defined as
Pure substances
A substance in which all of the particles that make up the substance are of exactly the same kind
two can physically combine to produce a mixture
Mixture
not chemically bonded
can be separated by physical means
in an element
atoms may be single or bound but all the same type
in a compound
at least two different types of atoms bound together in a specific ratio
in a mixture:
can be made of any combination of elements and and compounds and are not bonded to one another
not chemically bonded and be separated by physical means
Heterogeneous
not uniform throughout
homogeneous
uniform throughout
a solution
Aqueous Solution
a solution in water
Examples of Pure Substances
Elements & Compounds
hydrogen & sodium (element)
Table Salt Water (Compound)
Examples of Mixtures
Heterogeneous and Homogeneous Mixtures
oil & water, chicken noodle soup (hetero)
brass and vodka (homo)
Every substance has a definite set of
Properties
Propeties
characteristics by which something can be identified
Physical Properties
unrelated to changes in chemical composition
color, conductivity, & melting point
Chemical Properties
Describes the characteristic ways that a substance can be identified
related to how it reacts chemically with another substance
reactivity
Properties notes
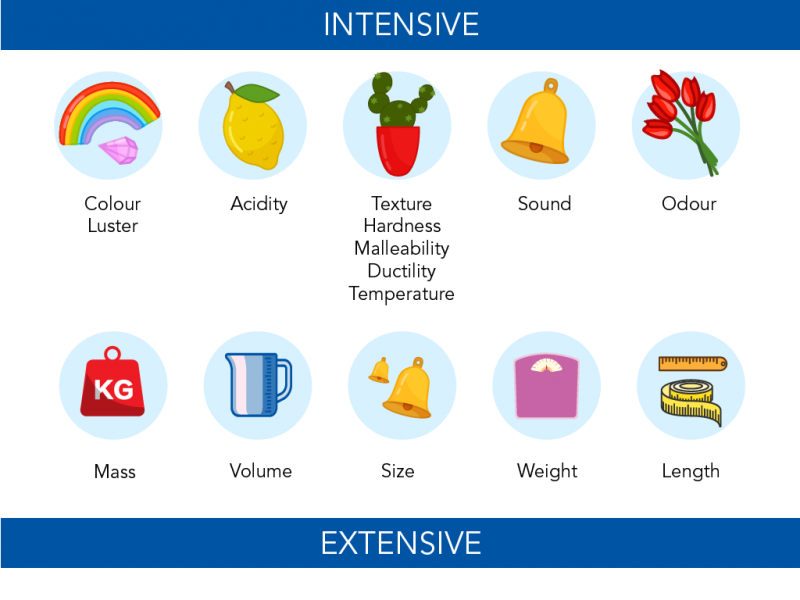
chemists distinguish properties that are extensive or intensive
properties of compounds are constant and different from the elements there made of.
when matter undergoes change, the starting material is the reactant and the resulting material is the product.
Extensive Properties
Depends of the Quantity
weight
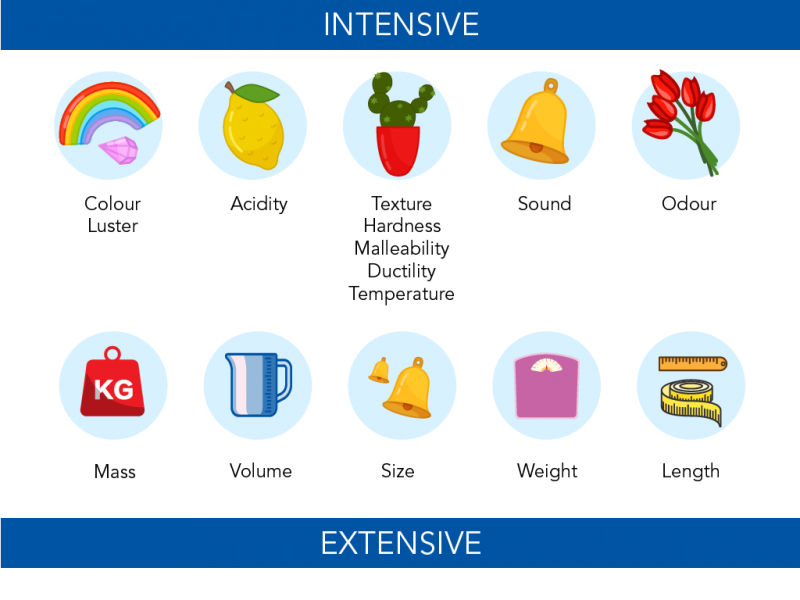
Intensive Properties
remains unchanged regardless of the quantity
color
boiling point
Physical Change
does not alter the chemical composition
boiling water changes the phase
separating a mixture into components ( a mixture of elements and compound)
might be separated into pure substances but the pure substances that result are the same ones that were in the mixture to begin with,
no pure substances were formed by separating a mixture into its components