1.3B Electron Orbitals
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
What equation tells us the maximum number of electrons that can be found in each energy level?
2n²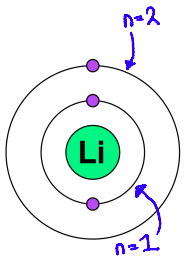
Define sub-level. How do you relate principal energy levels to sub-levels?
region where electron can be found and involves the shape of the region
Each principal energy level, n, can be divided into one or more sub-levels.
define Orbital
regions of space where there is a high probability of finding an electron
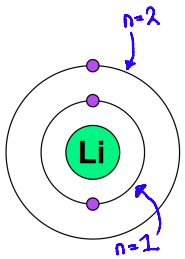
Tell me about the s sub-level using hydrogen and lithium as examples
spherical region close to the nucleus
Hydrogen
The hydrogen atom has one electron
This region is denoted 1s
Lithium
Lithium has an electron in n = 2
This electron inhabits a region known as 2s
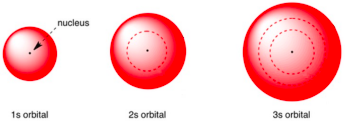
Define Node
a region where there is zero probability of finding an electron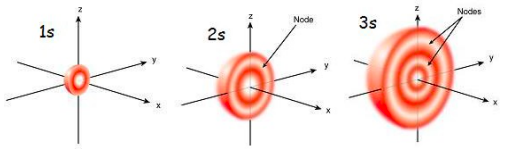
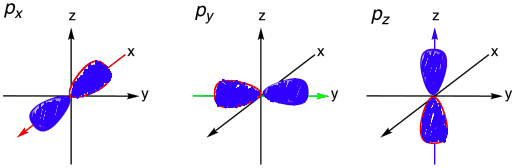
Tell me about the p sub-level
The second energy level, n = 2, can have s and p sub-levels
This sub-level is dumbbell shaped and it can point in different directions
px, py, and pz orbitals
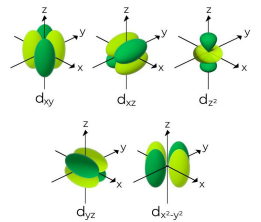
Tell me about the d sub-level
More energy levels means that the regions for an electron take on more shapes
Energy level n = 3 can have s, p, and d sublevels
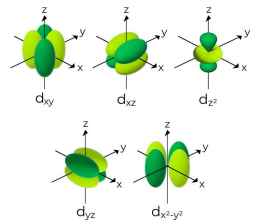
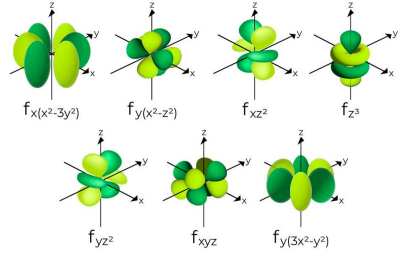
Tell me about the f sub-level
Energy level n = 4 and higher can have s, p , d, and f sub-levels