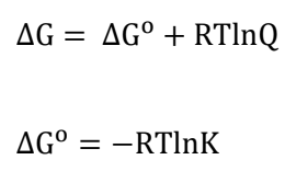Chapter 18 | Thermodynamics
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Last updated 2:23 AM on 8/16/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
1
New cards
Phase change entropy
Ssolid < Sliquid << Sgas
2
New cards
Standard entropy of reaction (ΔS0rxn)
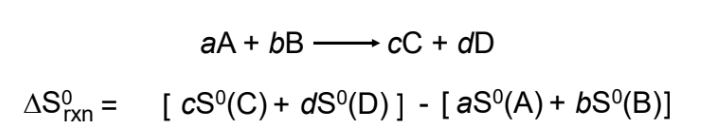
3
New cards
Gibbs Free Energy (G)
ΔG = ΔH -TΔS
ΔG < 0 The reaction is spontaneous in the forward direction
ΔG > 0 The reaction is nonspontaneous as written; the reaction is spontaneous in the reverse direction
ΔG = 0 The reaction is at equilibrium
4
New cards
Standard enthalpy of formation (ΔH0rxn)
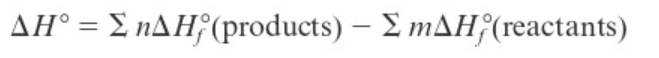
5
New cards
ΔG∘rxn
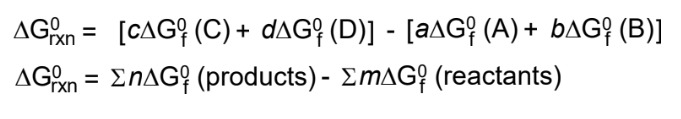
6
New cards
Gibbs Free Energy and Chemical Equilibrium