2Physiology of gaseous exchange and mechanisms of ventilation
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
What does the oxygen cascade describe?
How oxygen levels drop from the site of binding to haemoglobin to delivery at the tissues
What is the relationship between PaO₂ and SpO₂?
Essentially interchangeable
Majority of oxygen transport occurs via haemoglobin
What systems make up the body’s oxygen delivery system?
Lungs
Cardiovascular system
What does Dalton’s Law state about the total pressure of a gas mixture?
Total pressure = sum of the partial pressures of all constituent gases
What is the partial pressure of a gas in a mixture? daltons law
The pressure the gas would exert if it alone occupied the volume of the mixture
How is the total pressure of a gas mixture calculated? daltons
By summing the partial pressures of each individual gas in the mixture
What does Henry’s Law state about gas solubility in a liquid?
The amount of gas dissolved in a liquid is directly proportional to the gas’s partial pressure in contact with the liquid
Gas tension = partial pressure of gas in equilibrium with the solution
What factors affect the solubility of a gas in a liquid?
Each gas-liquid pair has a specific proportionality constant (Henry’s constant)
Solubility is inversely proportional to temperature for a given partial pressure
Why is alveolar PO₂ lower than atmospheric PO₂?
Only ~15% of alveolar air is replaced with each breath
Explains why alveolar gas composition remains relatively constant
Why is arterial PO₂ (PaO₂) lower than alveolar PO₂?
Some pulmonary blood bypasses alveoli (shunting)
Reduces arterial oxygen tension compared to alveolar air
Why is dissolved O₂ and CO₂ in blood alone insufficient?
Amount dissolved is too low for adequate tissue O₂ delivery and CO₂ removal
Mechanisms exist to increase carrying capacity
How is the carrying capacity of O₂ and CO₂ increased in blood?
O₂: bound to haemoglobin (~70× more than dissolved)
CO₂: converted to bicarbonate and other forms (~17× more than dissolved)
Why are PO₂ and PCO₂ important despite these mechanisms?
Determine how much gas is carried by haemoglobin and other systems
Drive gas transfer in alveoli and tissues via partial pressure differences
Dissolved O₂ important in therapies like hyperbaric 100% O₂ for CO poisoning
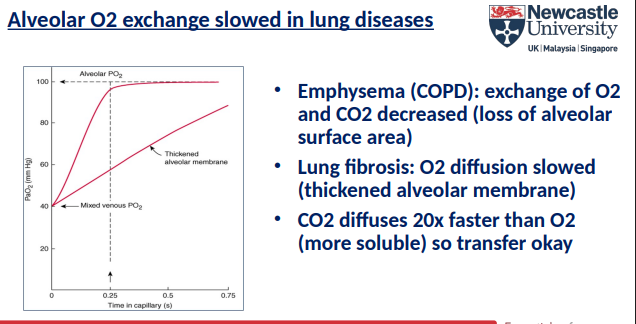
How quickly does gas diffusion occur between alveoli and blood?
Pulmonary capillary blood equilibrates rapidly with alveolar air
O₂ and CO₂ diffusion across alveoli is very fast
What factors determine the rate of gas diffusion across alveoli?
Large surface area
Thin membrane
Partial pressure differences (alveolar ↔ blood)
Gas solubility