Organic Chemistry 💀
1/45
Earn XP
Description and Tags
Notes: oxygen is king in naming order, numbers are separated by commas, letters and numbers are separated by dashes.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
Main/parent chain
the longest continuous chain of carbon atoms in a compound
Structural isomers
molecules with identical empirical/molecular formulas, but with a different arrangement of atoms
Alkane
hydrocarbon compounds consisting of all single C-C bonds
general formula: CnH2n+2
Substituent
an atom or group of atoms that replaces a hydrogen atom/atoms on a carbon chain
Alkyl group
a substituent that consists of only carbon and hydrogen (e.g. a methyl group)
Alkene
hydrocarbon compounds containing one or more double C-C bonds
general formula: CnH2n
Alkynes
hydrocarbon compounds containing one or more triple C-C bonds
general formula: CnH2n-2
Functional groups
substituents which change the chemical properties of organic compounds
Halogenoalkane (halide)
carbon chain with F, Cl, Br, or I attached.
R-X
Ex: chloromethane
Alcohol (hydroxyl)
carbon chain with OH attached.
R-OH
Ex: propanol
Aldehyde (carbonyl)
carbon chain with C bonded to O and H at the end.
RCHO
Ex: ethanal
Ketone
carbon chain with C bonded to O somewhere in the middle.
R-COR’
Ex: Pentanone
Carboxylic acids (carboxyl)
carbon chain with C bonded to O and OH at the end.
R-COOH
Eg. ethanoic acid
Homologous series
different molecules that are members of the same chemical family (e.g. alkanes…) that differ from each other only by the number of CH2 groups.
Trends - as chain length increases so does boiling point
Amine (amino)
carbon chain with NH2 at the end or NH in the middle (for both options N has a lone pair)
R-NH2 or R-N-R’
Ex. methylamine (H3CNH2)
Amide (amido)
carbon chain with C bonded to O and NH in the middle.
R-CO-N-R’
Arene (phenyl)
carbon chain bonded to ring of 6 Cs and 5 Hs
R-C6H5
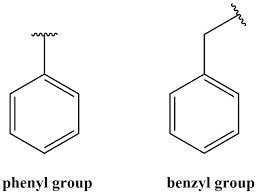
Ester (ester)
carbon chain with C bonded to O bonded to O which is bonded to the rest of the chain.
R-COO-R’
Ether (alkoxy)
carbon chain with O in the middle (O has two lone pairs)
R-O-R
Nucleophile
a chemical species that is attracted to positive charges, can donate a pair of electrons
Ex. ligands, polar molecules, anions
Electrophile
a chemical species that is attracted to negative charges
Ex. cations
Free radical
highly reactive species due to the presence of an unpaired valence electrons. Unpaired electrons are shown as a dot
Ex. Cl•
Homolytic fission
the breaking of a covalent bond where each of the bonding species take one of the bonding electrons each
ex. Cl2 → Cl• + Cl•
Heterolytic fission
the breaking of a covalent bond where one of the bonding species takes both of the bonding electrons
ex. Cl2 → Cl- + Cl+
Substitution
one species replaces another in an organic molecule
Addition
two or more molecules combine
Oxidation
characterized by a loss of electrons OR the gain of oxygen and/or the loss of hydrogen
Reduction
characterized by a gain of electrons OR the loss of oxygen and/or the gain of hydrogen
Combustion equations
Complete: fuel + O2 → CO2 + H2O
Incomplete: fuel + O2 → CO + C + H2O
Free radical nucleophilic substitution (steps)
Initiation: UV radiation supplies the energy needed to initiate homolytic bond fission in the halogen, creating free radicals
Cl2 → 2Cl•
Propagation: the free radicals are strong nucleophiles and will react with the alkane to produce and alkyl radical and a hydrogen halide
CH4 + Cl• → CH3• + HCl
CH3• + Cl2 → CH3Cl + Cl•
Termination: free radicals produced will react with one another
Cl• + Cl• → Cl2 OR CH3• + Cl• → CH3Cl
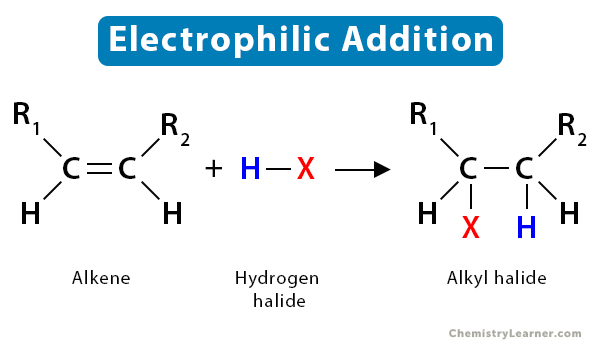
Electrophilic addition
occurs in alkenes and alkynes because the double bond breaks so each carbon can bond to one additional substituent.
Nucleophilic substitution
the nucleophile replaces the halogen, creating a new compound and a halide ion
Ex. C2H5Br+ OH1- → C2H5OH + Br(dot)
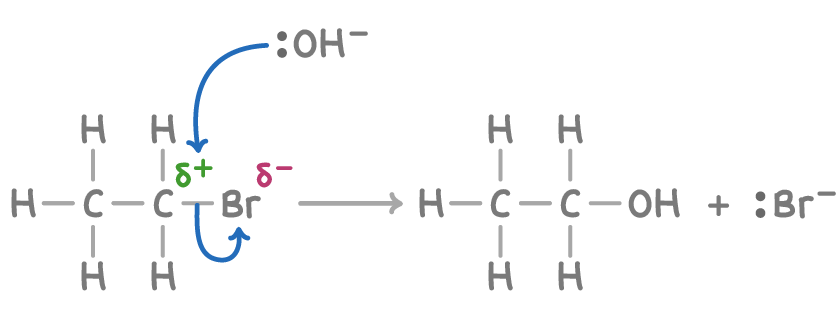
Addition polymerization
the double bond breaks & the C originally with the double bond now bonds to 2 new things
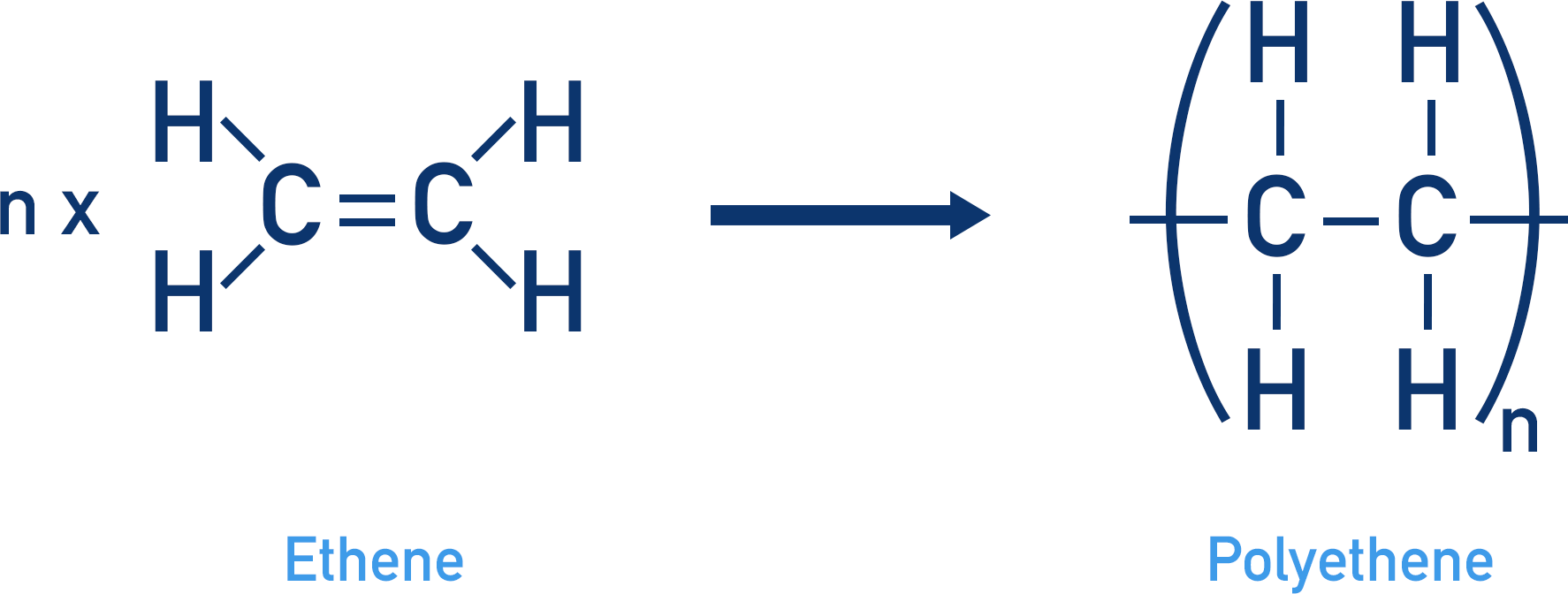
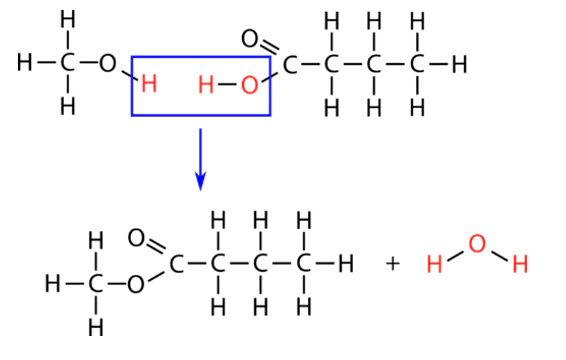
Condensation polymerization
the reacting molecules and product molecule have functional groups on the ends. Smaller molecules (often H2O) are produced.
Diols - molecules with hydroxyl groups on each end (OH)
Dioc acids - molecules with carboxyl groups on each end (COOH)
Diamines - molecules with amino groups on each end (NH2)
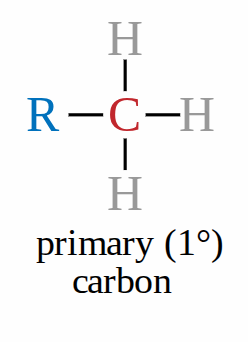
Primary carbon
when the carbon bonded to the functional group is also attached to 1 carbon
Secondary carbon
when the carbon bonded to the functional group is also attached to 2 carbons
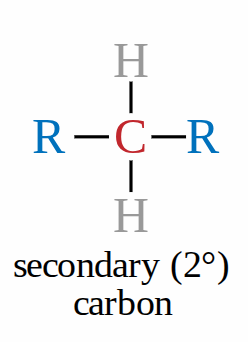
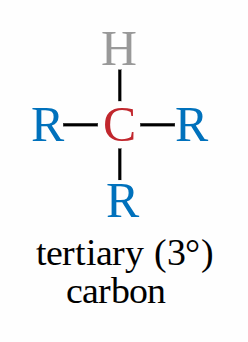
Tertiary carbon
when the carbon bonded to the functional group is also attached to 3 carbons
Oxidation of primary alcohols
occurs in two oxidation steps: the first step produces an aldehyde through distillation, if the second step is allowed to occur, a carboxylic acid is produced through reflux.
CxHy + [O] → CxHy with CHO or COOH
Oxidation of secondary alcohols
occurs in one oxidation step which produces a ketone.
Oxidation of tertiary alcohols
cannot be oxidized
Reduction of organic compounds
Carboxylic acids → reduced to aldehydes using lithium aluminium hydride (Li[AlH₄])
Aldehydes → reduced to primary alcohols using sodium borohydride (NaBH₄)
Ketones → reduced to secondary alcohols using sodium borohydride (NaBH₄)
Saturation
When each carbon atom in an organic compound is bonded to the maximum number of hydrogen atoms (4)
Formula for IHD
(0.5)(2C + 2 - H - X + N), where X is a bonded halogen
Integration trace
The ratio of hydrogens in each environment (signal)
The splitting tells us…
How many hydrogens are on the adjacent carbon (if one hydrogen, its a doublet… so on)
X-ray crystallography
a technique that uses reflected x-rays to identify bond lengths and angles in crystal structures