Chemistry Quiz 6 Electron/Molecular Geometry Practice
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
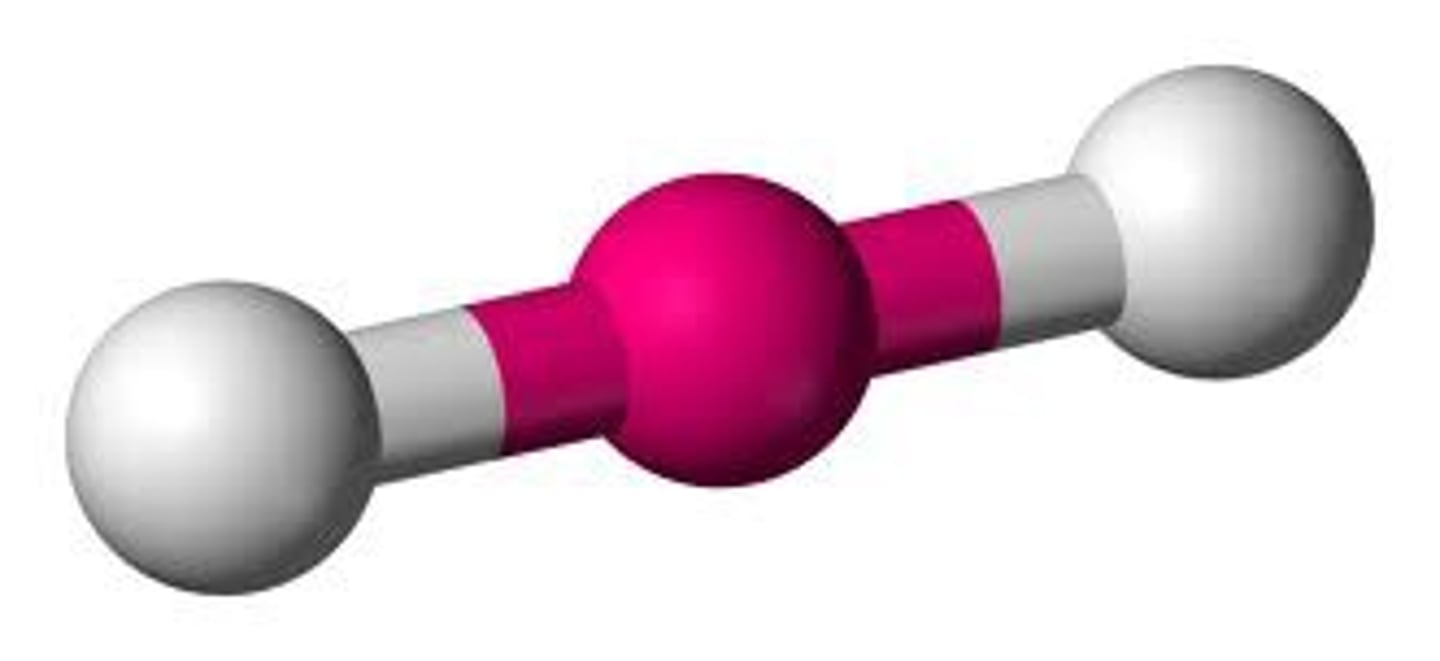
Electron groups: 2 Lone Pairs: 0
Electron Geometry: Linear, Molecular Geometry: Linear
180˚
Electron groups: 3 Lone pairs: 0
Electron Geometry: trigonal planar, Molecular Geometry: trigonal planar
120˚
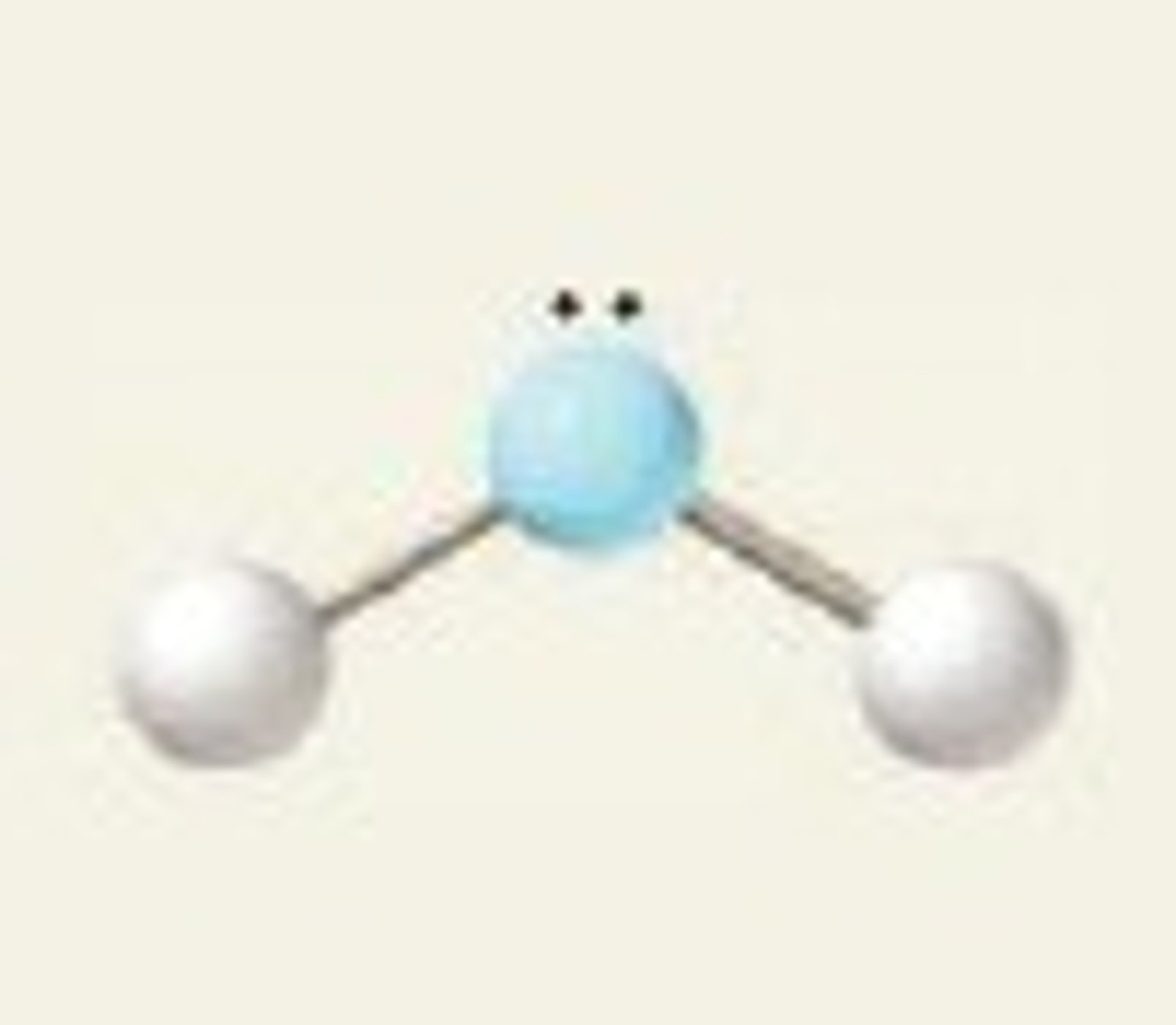
Electron groups: 3 Lone pairs: 1
Electron Geometry: trigonal planar, Molecular Geometry: bent
<120˚
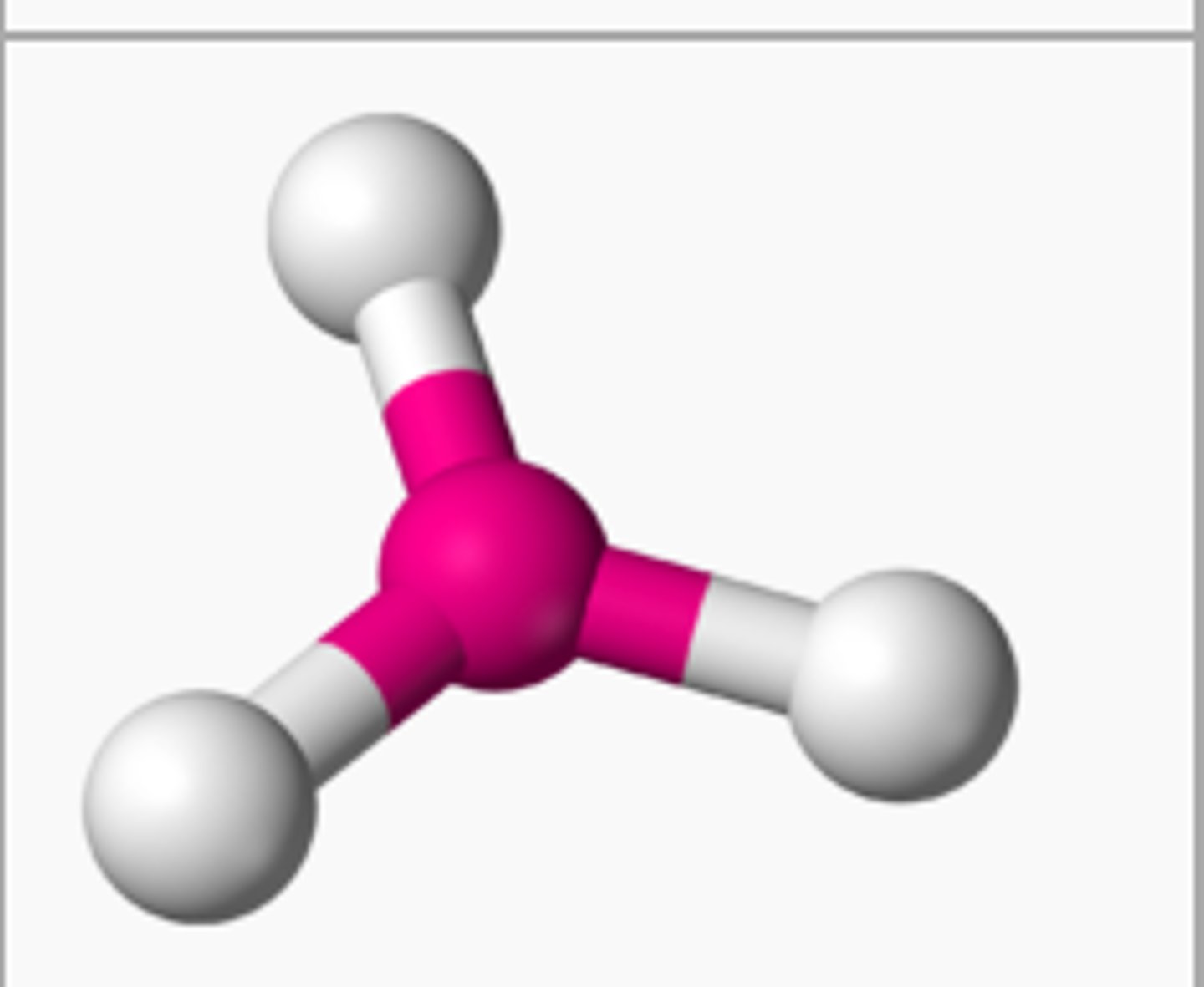
Electron groups: 4 Lone pairs: 0
Electron Geometry: tetrahedral, Molecular Geometry: tetrahedral
109.5˚
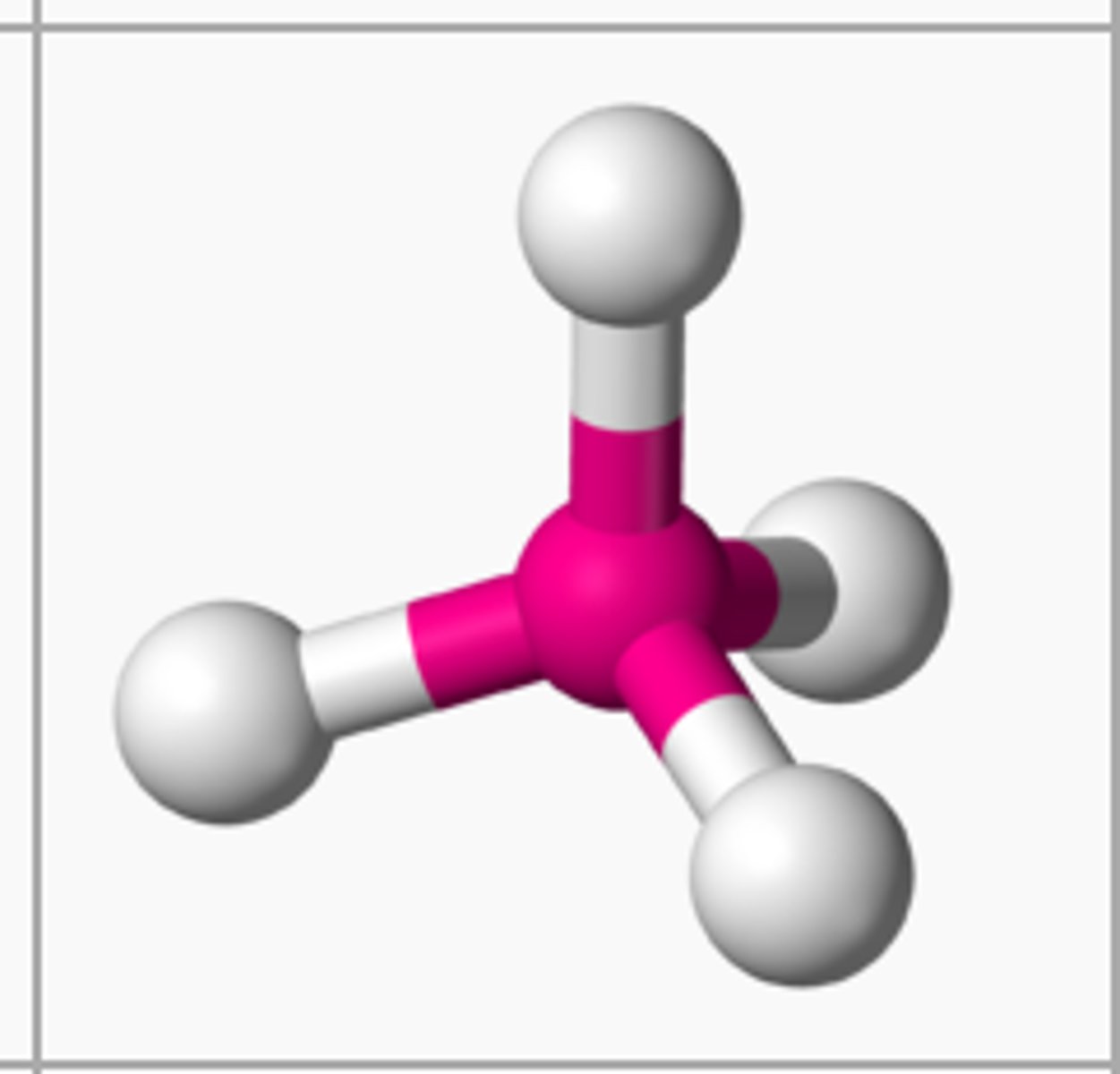
electron groups: 4 Lone pairs: 1
Electron Geometry: tetrahedral, Molecular Geometry: trigonal pyramidal
<109.5
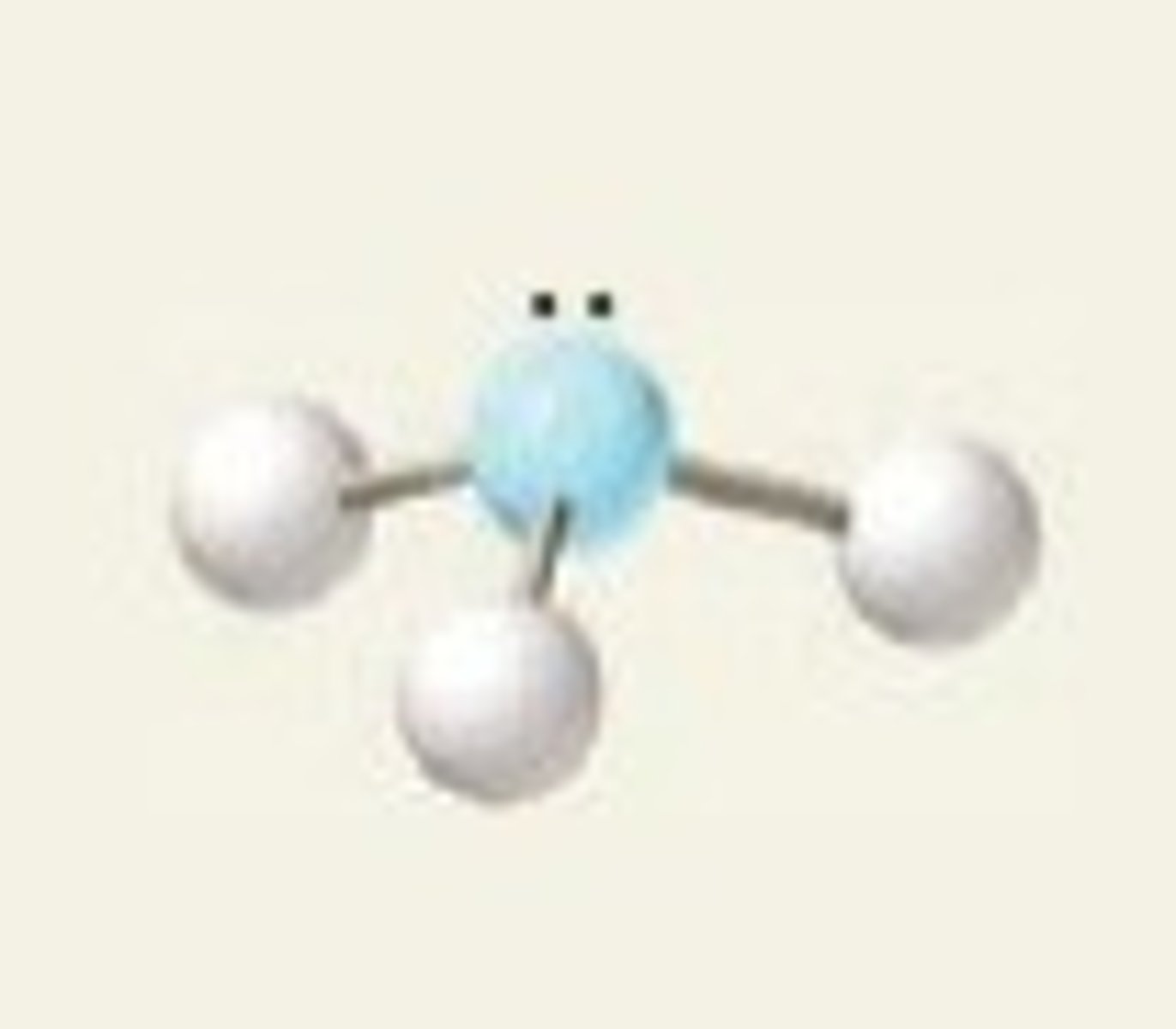
Electron groups: 4 Lone pairs: 2
Electron Geometry: tetrahedral, Molecular Geometry: bent
<<109.5˚
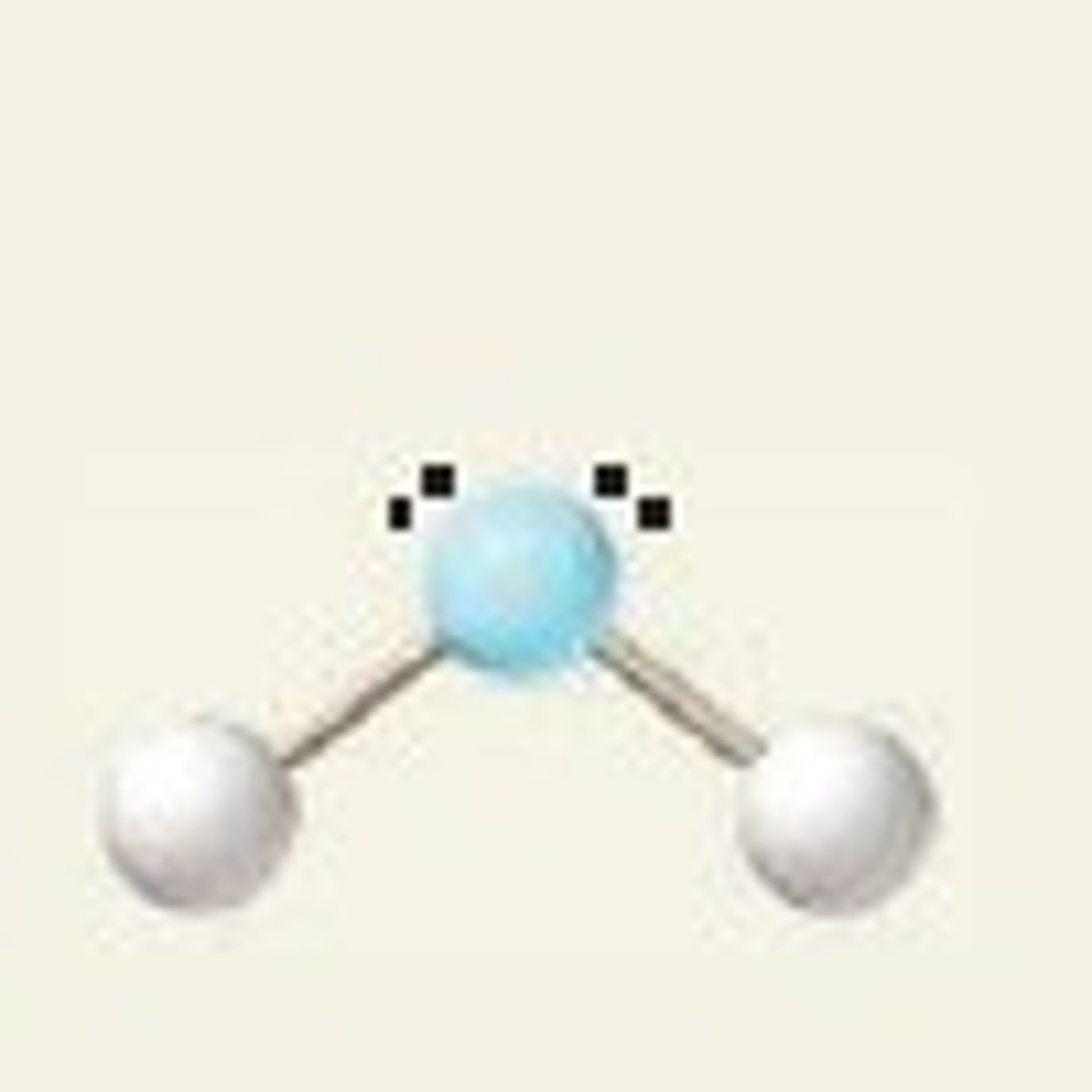
Electron groups: 5 Lone pairs: 0
Electron Geometry: trigonal bipyramidal, Molecular Geometry: trigonal bipyramidal
120˚ (equatorial), 90˚ (axial)
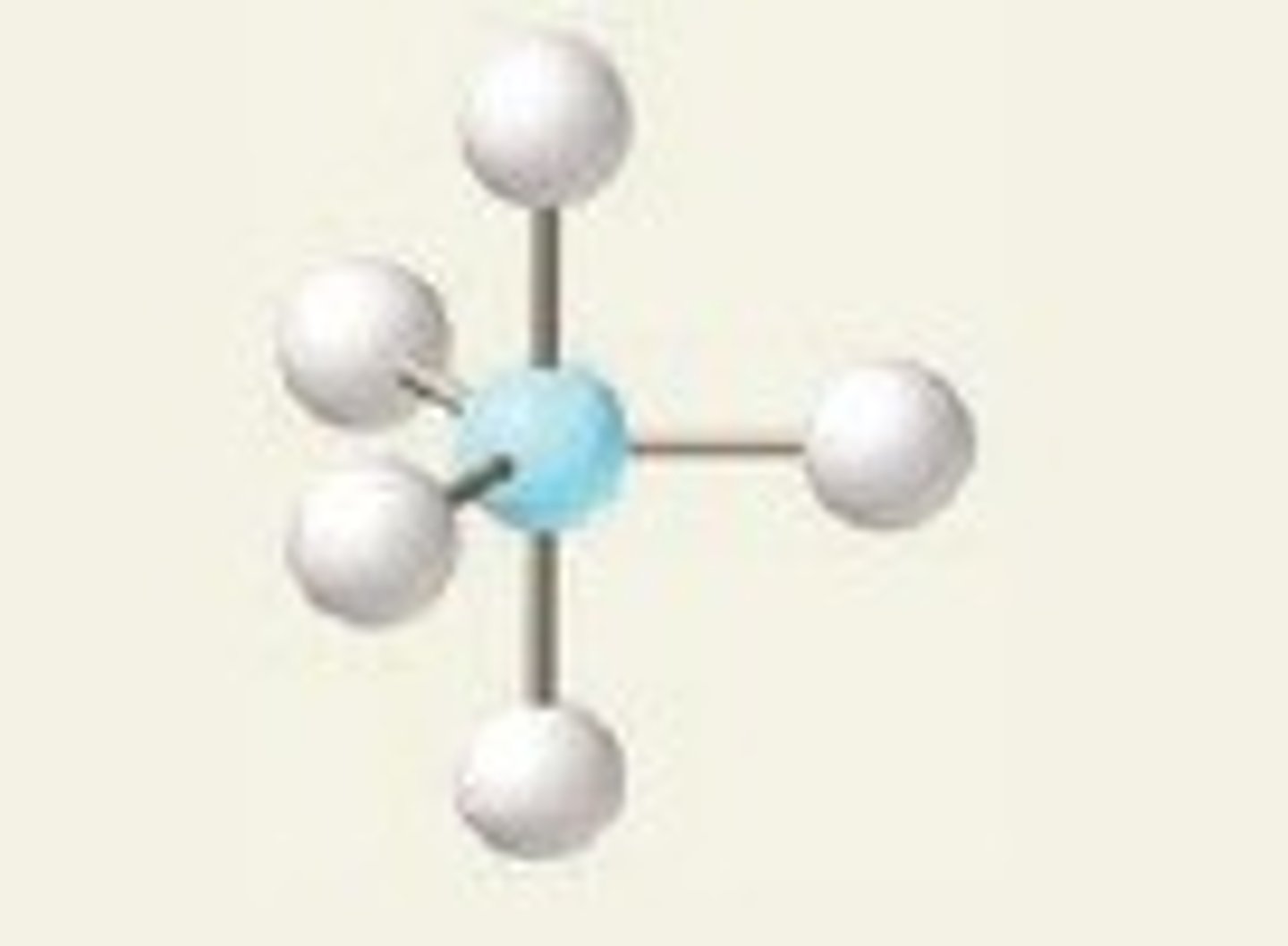
Electron groups: 5 Lone pairs: 1
Electron Geometry: trigonal pyramidal, Molecular Geometry: Seesaw
<120˚ (equatorial)
<90˚ (axial)
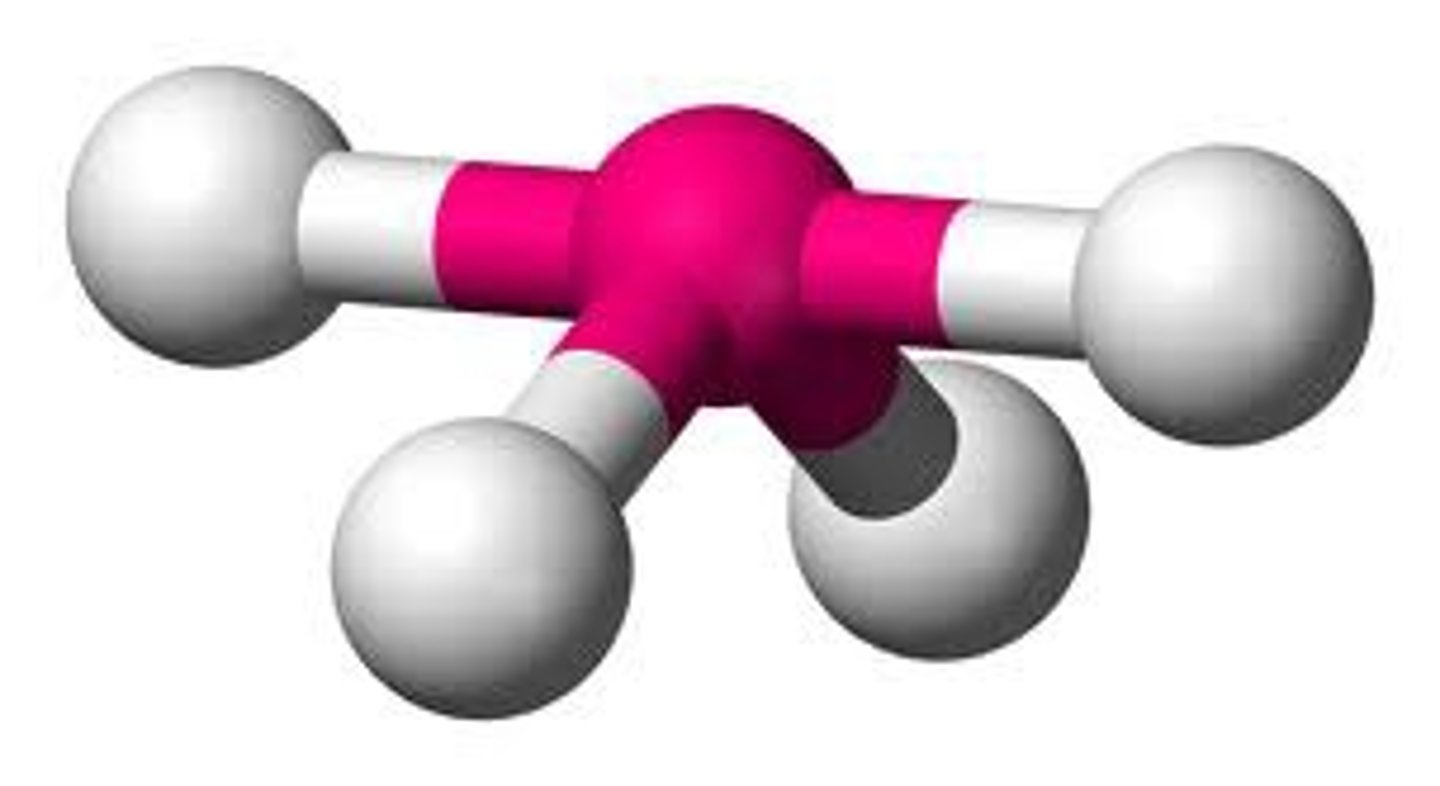
Electron groups: 5 Lone Pairs: 2
Electron Geometry: trigonal pyramidal, Molecular Geometry: T-shape
<90˚
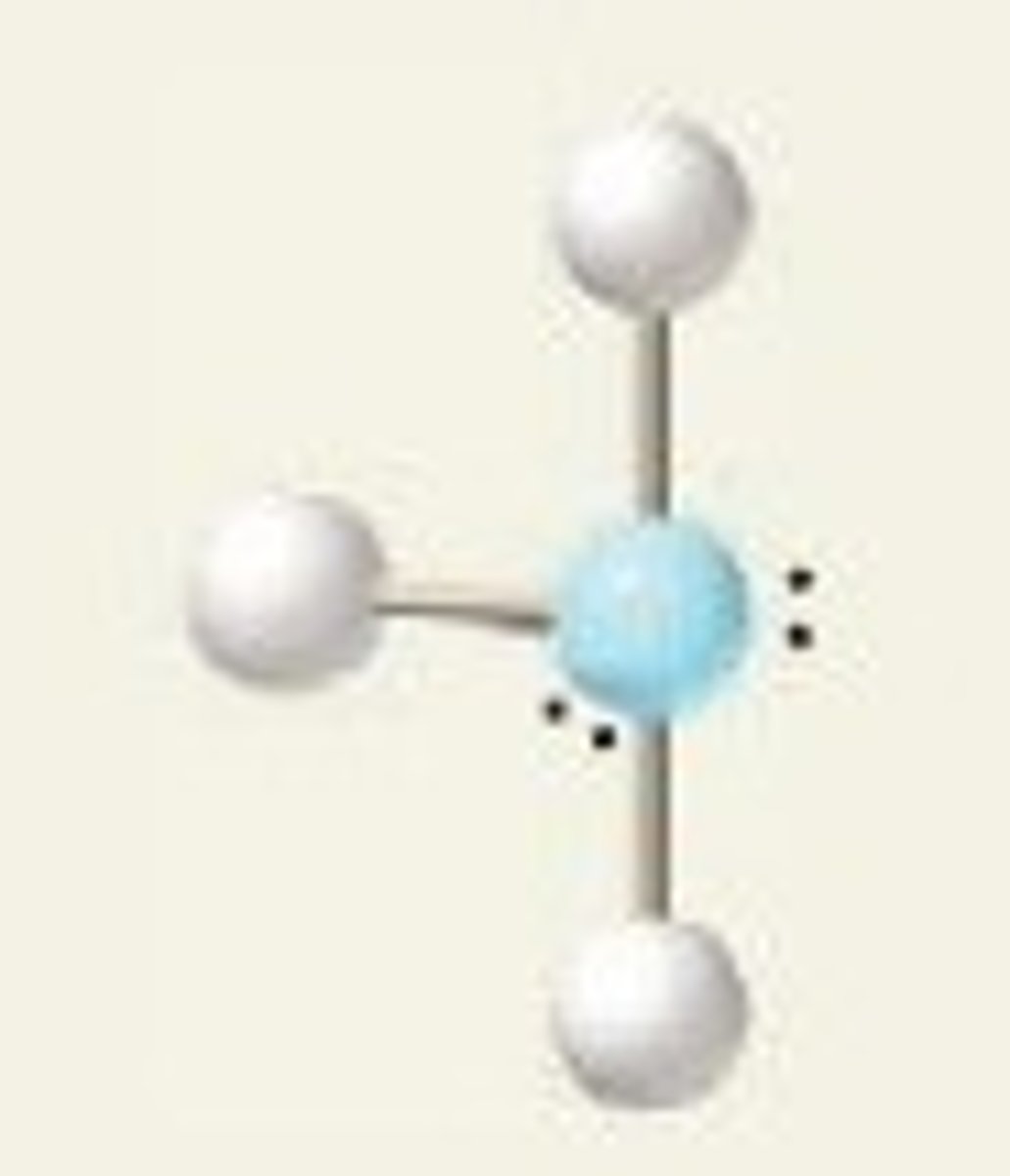
Electron groups: 6 Lone Pairs: 0
Electron Geometry: Octahedral Molecular Geometry: Octahedral
90˚
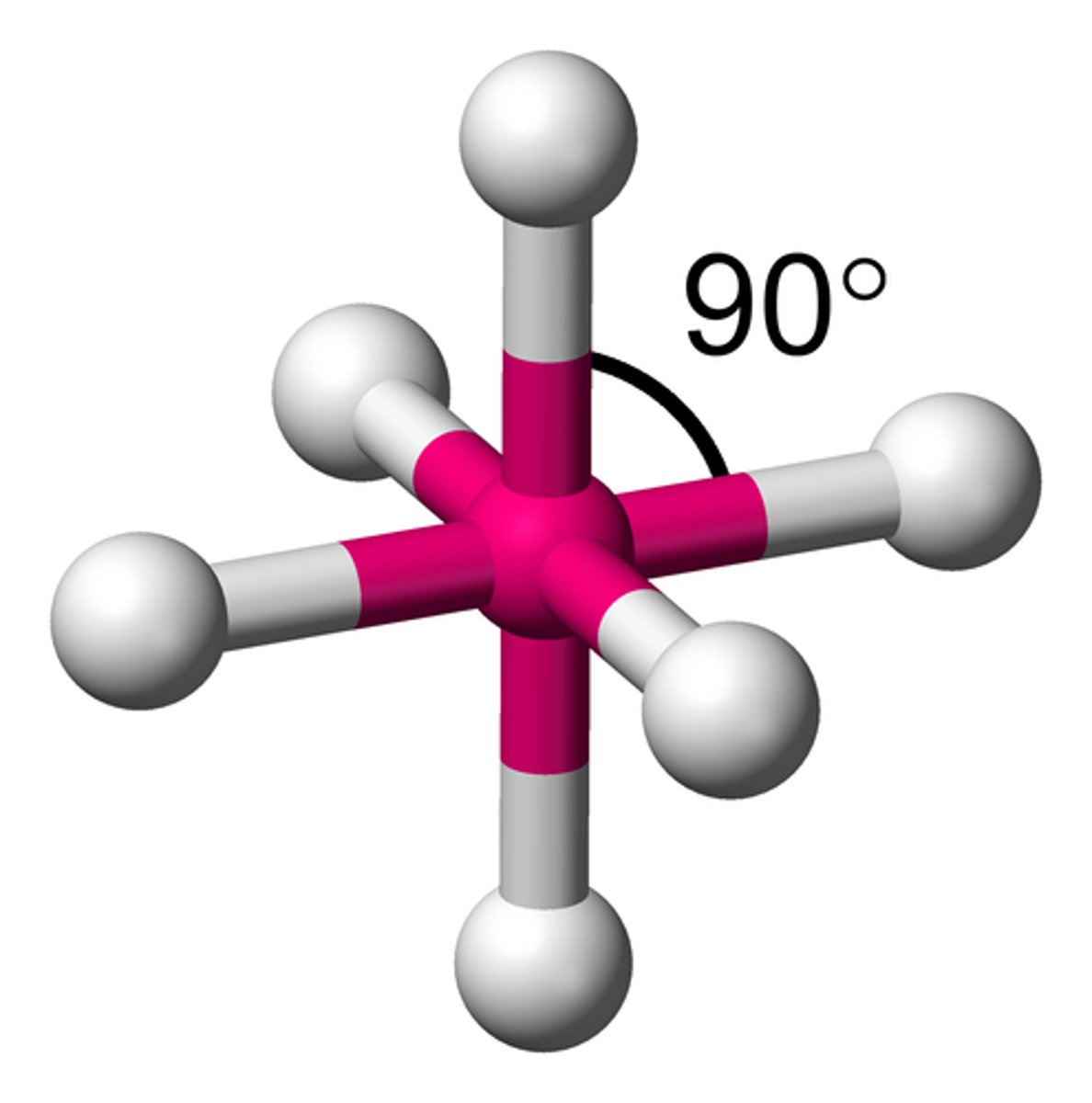
Electron groups: 6 Lone Pairs: 1
Electron Geometry: Octahedral Molecular Geometry: Square Pyramidal
<90˚
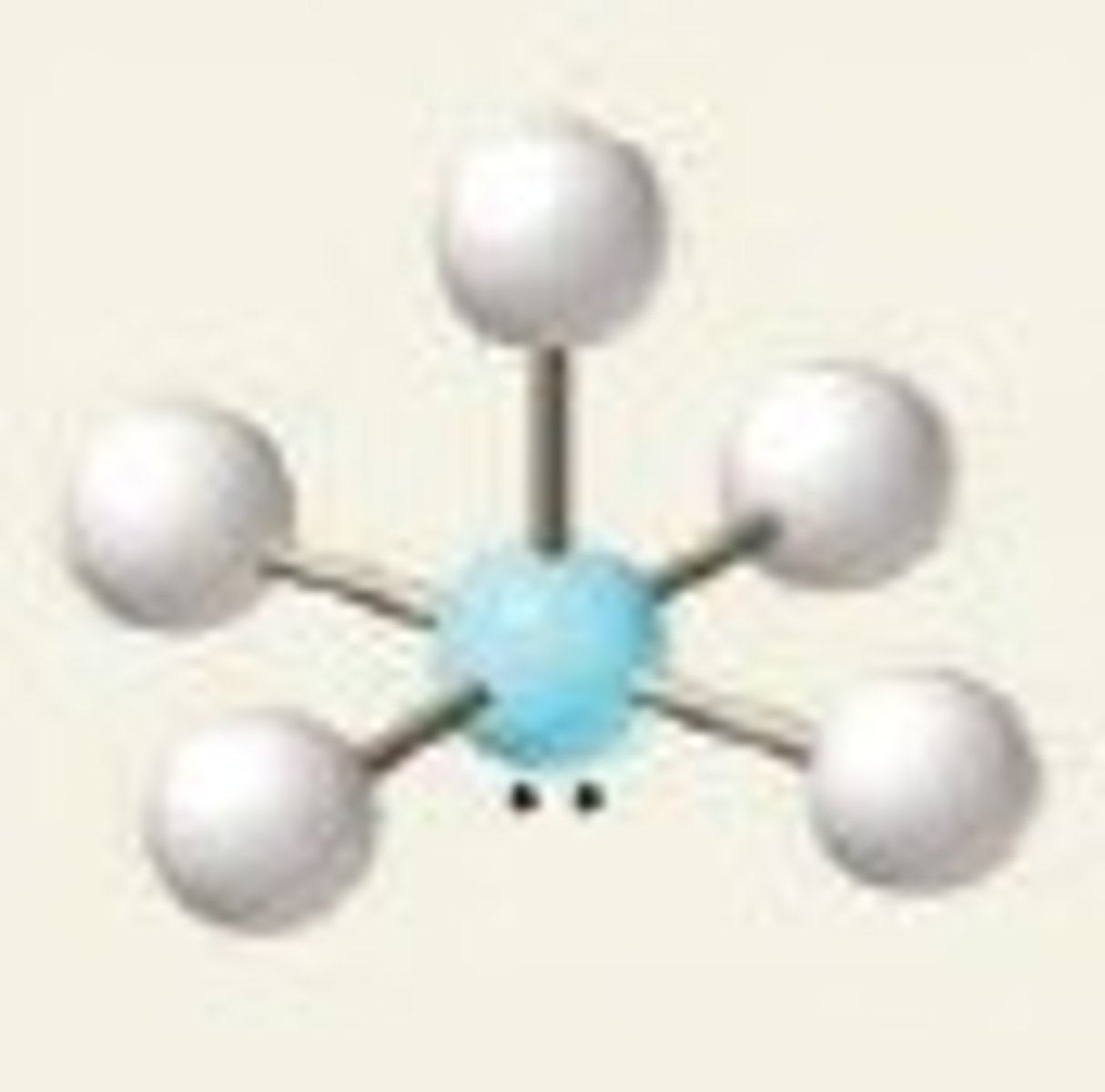
Electron groups: 6 Lone Pairs: 2
Electron Geometry: Octahedral, Molecular Geometry: Square Planar
90˚
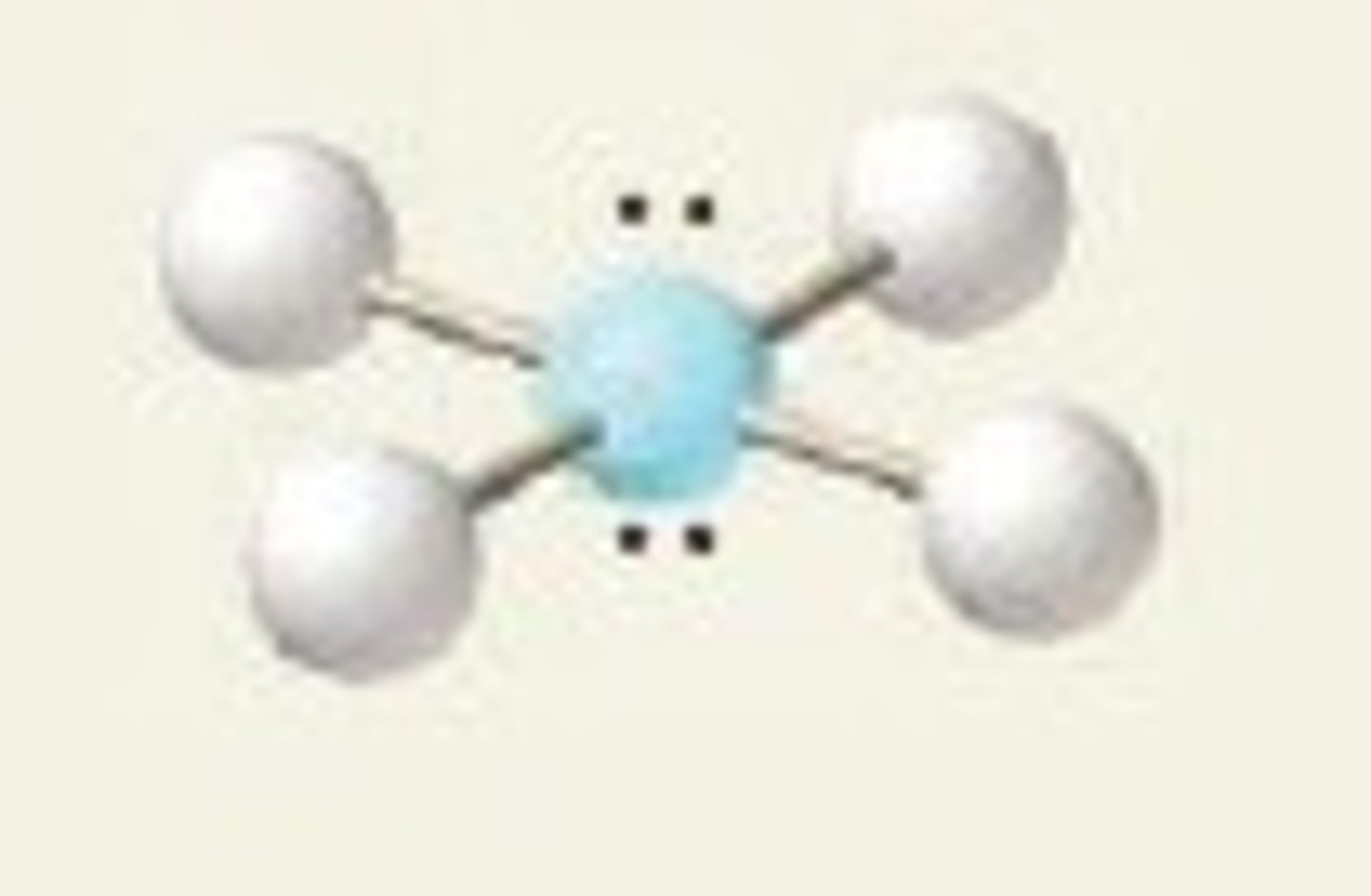
AlCl3
Electron Groups:5 Lone Pairs: 2
Electron geometry: trigonal bipyramidal Molecular Geometry: trigonal planar
The same applies to BF3