Periodic Table TEST REVIEW!!!
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
Subatomic Particles in the Nucleus of an Atom
Protons and Neutrons
How do you caculate the number of neutrons?
Mass # - Atomic #
How do you calculate Mass # of an Atom
Protons in Nucleus + Neutrons in Nucleus
What did John Newlands discover
Law of Octaves- When arranged by atomic mass, each element was similar to element eight places further on periodic table
Mendeleev discovery
First periodic table-Arranged elements in order of increasing atomic mass (WRONG)
Moseley Discovered
Current periodic table. Elements arranged in order of Increasing Atomic #, Theres a periodic repetition of their phys. and chem properties. (Think name- Mo=Modern)
How many periods? (horizontal row)
7
Whats group? (Vertical column)
Similar phys. and chem. properties identified by number and letter
Three classes of elements are:
Metal, Metalloid, Nonmetal
What side of stair step are metals on?
Left
What side of the stair step are nonmetals?
Right. Includes hydrogen despite deciding whether its a metalloid currently
Where are Metalloid properties?
Intermediate between metalss and nonmetals. Border the stair step
Is aluminum a metalloid? What is it?
No. Its a metal.
Whats a name for Group 1A
Alkali metals. Violent reaction w/ water
Whats a name for Group 2A
Alkaline Earth Metals. Form bases w/ water. Do not dissolve well. “Earth Metals”
Name for Group 7A
Halogens
What does Halogens mean?
Salt-forming
What group are Noble Gases in?
8A or Group 0. Very stable. Also called inert gases.
What are elements in the 1A-7A groups called?
The Representitive Elements
What Group columns are Transition metals in?
Group B. Outer s sublevel full, now filling the d sublevel. Transition between metal and nonmetal area
Inner Transition Metals are located where?
Below the main body of the table in 2 horizontal rows. Has outer s sublevel full and is now filling F sublevel
Why are the Inner Transition Metals no longer called “Rare Earth” elements?
Because some are very abundant
Name atleast 3 properties of Metals
Options: High luster, Malleable, Ductile, conductor, located on left side stair step
Name atleast 3 properties of NonMetals
Options: Located on right side of stair step including hydrogen, brittle, poor luster, poor conductors
Anthinide Series, Actinide Series
6, 7!!
Electrons in an atom are in constant motion in what is known as an:
Electron Cloud
What do we use to measure where an electron is?
Light
Heisenberg Uncertainty Principle:
One cannot sinulatenously determine both the position and momentum of an electron
What is 𝜆=ℎ/𝑚𝑣
De Broglie’s Equation, calculates wavelength of a particle. H is plancks constant, m is mass, v is velocity. Plancks constant is 6.62×10^-34
Whats shell (energy level)
Region around nucleus of atom where an electron is likely moving

Know this image:
Whats an Orbital?
Regions around nucleus of atom w/ high probability of finding an electron. KNOW THE IMAGE
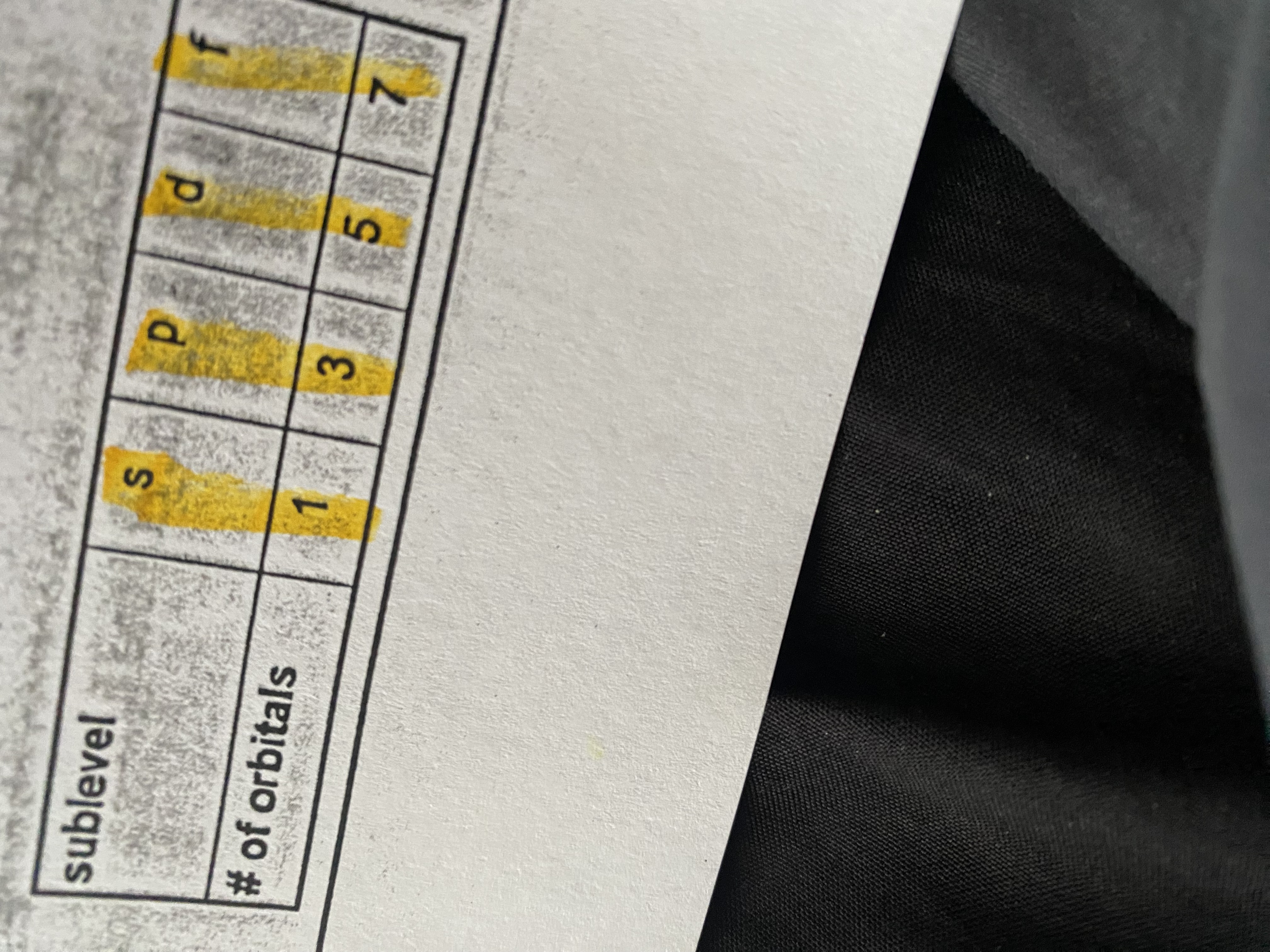
How to calculate max # of electrons in an energy level?
2(n)² or 2(energy level)²

Know this image:
How many electrons can each energy level hold?
s=2 p=6 d=10 f=14
J.W Dobereiner did what?
Grouped elements into triads
Broglie proposed what
Wave-particle duality
Group stored under oil:
Alkali Metals (Group 1A)
how many Electrons in 4th energy level
32
How many orbitals in 3rd energy levels?
9
Transition metals have what sublevel filling?
d
Are noble gasses nonmetal? Are they all gasses?
True, True