Condensation Polymerisation
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
When does condensation polymerisation happen?
monomers of different functional groups to form bonds and give polymer chains where a small molecule is lost so atom economy is not 100%
What is the small molecule that is always eliminated in the natural condensation polymerisation?
water
How can you identify condensation polymers?
ester/amide link
Diols
have -OH molecules on either side
General formula for diols
HOCnH2nOH
Pentane diol
HOC5H10OH
Diamines
have NH2 on either side
general formula for diamines
H2NCnH2nNH2
Ethane Diamine
H2NC2H4NH2
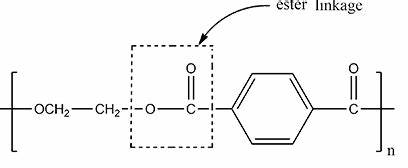
ester link
link diols and dicarboxylic acids
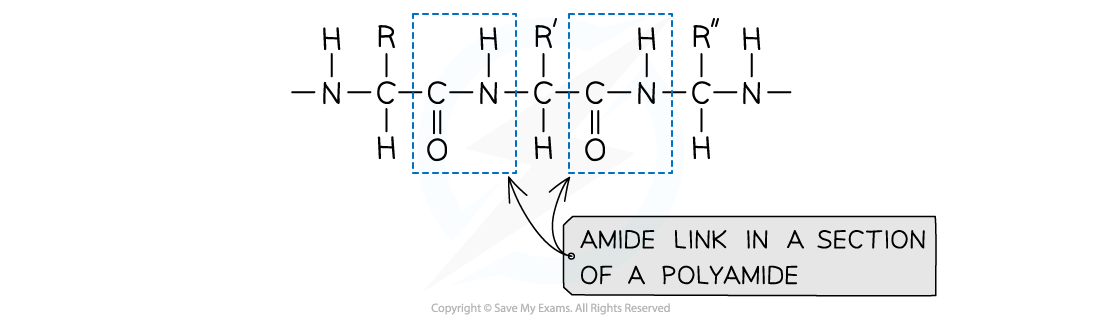
amide link
link diamines and dicarboxylic acids
Polyesters
formed by linking dicarboxylic acids and diols by ester link