further aspects of covalent bonding
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
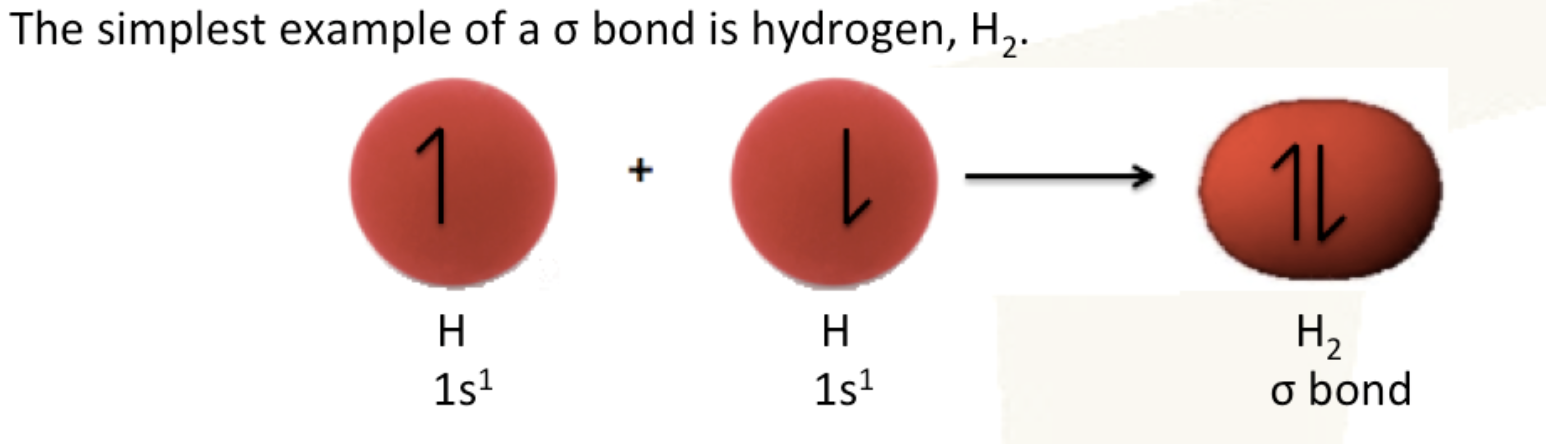
how sigma bonds are formed
formed by the direct head-on/end-to-end overlap of atomic orbitals; combine along a line drawn through the two nuclei to form a new molecular orbital. This results in the electron density concentrated between the nuclei of the bonding atoms
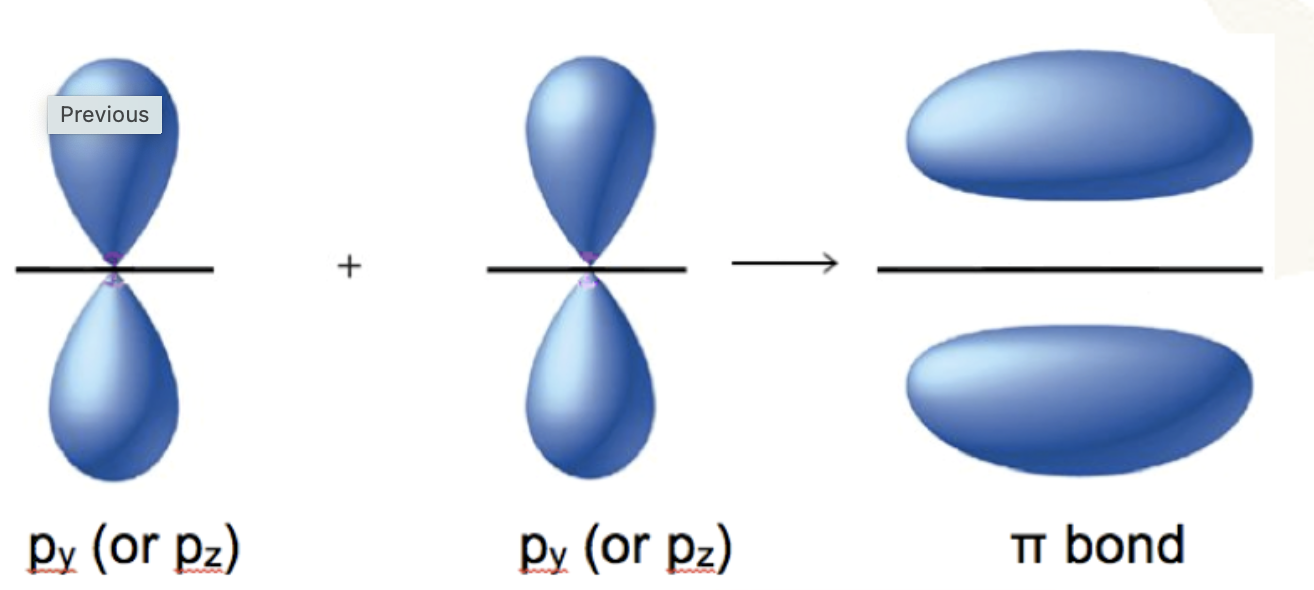
how pi bonds are formed
formed by the sideways overlap of atomic orbitals. This results in the electron density being above and below the plane of the nuclei of the bonding atoms.
how to find preferred lewis structure
FC = (Number of valence electrons) − ½(Number of bonding electrons) − (Number of non-bonding electrons).
The preferred Lewis structure is the one with the atoms having formal charge values closest to zero.
formal charge
charge an atom would have if all atoms in the molecule had the same electronegativity
delocalisation
When electrons are shared by, or between, more than one pair of atoms in a molecule or ion (as opposed to the electrons being localized between just two of atoms).
resonance structures
one of two or more alternative Lewis structures for a molecule or ion that cannot be described fully with one Lewis structure alone.
what a single bond is made up of
one sigma bond
what a double bond is made up of
one sigma bond and one pi bond
ozone and oxygen
ozone bond order= 1.5 due to delocalisation, o2 double bond, bond order= 2
→ therefore, ozone requires higher energy to break an oxygen-oxygen bond, but both require energy in the ultraviolet area of the spectrum.
ozone layer features
ozone and oxygen are in equilibrium, ozone is being continually formed and decomposed, absorbing uv light in the process
→ reduces the amount of low and high uv radiation reaching the earth surface
ozone depletion catalysed by cfcs
carbon - cl bonds are broken down in the presence of UV light to form radicals that can break down ozone and propagate more radicals
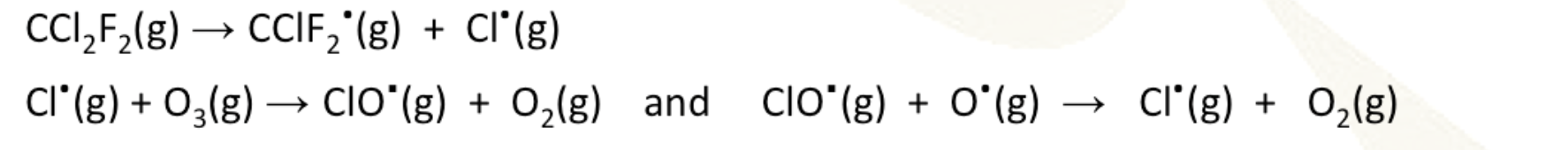
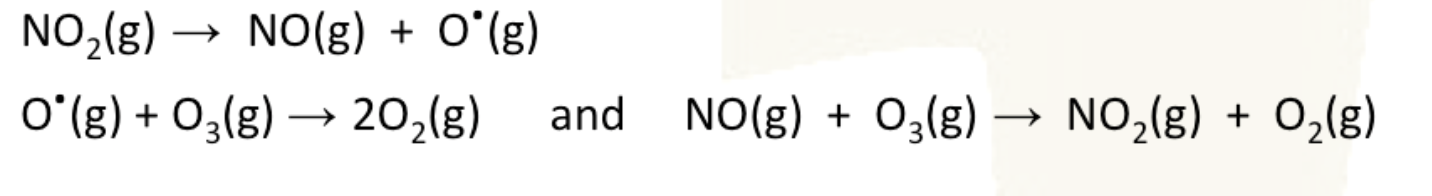
ozone depletion catalysed by NOx
both oxygen radical and nitrogen monoxide can decompose O3. the reaction with NO regenerates the catalyst