S2.3 Metallic bonding and structure
1/9
Earn XP
Description and Tags
Only Transition metal is Hl stuff
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
What is metallic bonding?
Metallic bonding is the electrostatic attraction between cations and delocalised valance electrons in the 3-D lattice.
Metallic bonding results from the electrostatic attraction between metal cations and the sea of delocalized electrons around them
Core electrons are not involved.
How are solid metals similar to ionic and covalent network substances?
all has solids form three-dimensional lattices.
all has electrostatic attractions between positive and negative species.
Metallic – between cations and delocalised electrons, ionic – between cations and anions, covalent – between positive nuclei and shared electron pair.
Ionic and metallic bonding have non-directional bonding, however, covalent bonding is directional.
State which of the following has metallic bonding:
MgO(s)
Mg(s)
Mg(g)
Mg2+(aq)
Mg(s)
Metallic bonding is the interaction between positive metal ions and delocalised valence electrons in a three-dimensional lattice structure. The metal itself is neutral and is made up of many, many atoms.
Mg2+ is incorrect because it is an individual charged ion surrounded by a water solvent. MgO is incorrect because it is an ionic compound.
Mg(g) is incorrect because a gas would involve only individual metal atoms that are too far apart to be bonded to one another.
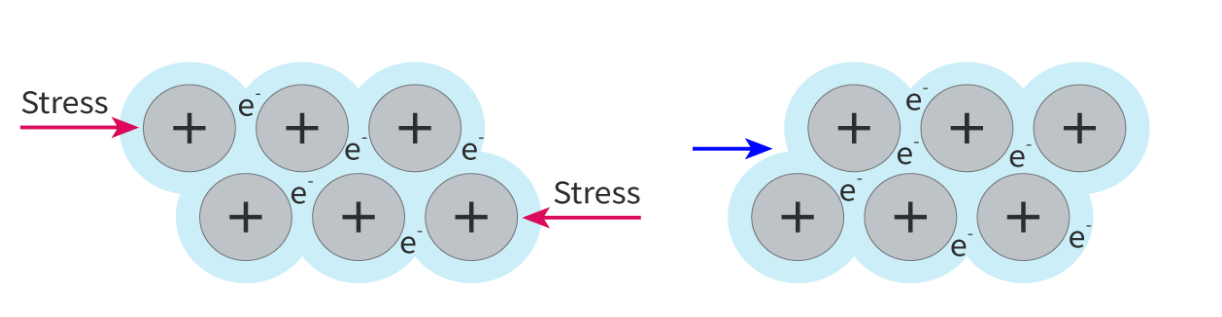
Explain the malleability ( ability to be bent and reshaped when compressed)and ductility (ability to be drawn out into a wire when stretched) of metals
Layers of cations can slide past each other without breaking the metallic bonds.
If stress is applied to the metal, planes of atoms slide over each other. This means the structure can change shape without the crystal fracturing. In contrast, stress applied to an ionic crystal will cause it to shatter.
(layers of positive ions can slide over each other without breaking more bonds that are made)
when a force is applied to a piece of metal, the layers of ions can slide over each other without affecting the bonding because metallic bonding is non directional. the metal ions in the lattice attract delocalised electrons in all directions and vice versa. so when one layer is displaced relative to another, the positive ions still attract the delocalised electrons in the same way.
Explain trends in melting points of Sand P block metals
strength of metallic bond < charge density< number of valance elctrons per atom/ metallic ion radius
<= directly proportional
Explain the high melting point of transition metals
Higher than s-block metals due to the stronger metallic bonding that results from the greater number of valance electrons and smaller ionic radii. The cations have a higher charge density and hence a stronger electrostatic attraction to the delocalized sea of electrons.
Explain the electrical conductivity (the ability of charged particles to move through a region of space) of transition metals.
These metals form their metallic bonds through the delocalisation of electrons in unfilled d orbitals.
When a potential energy difference is applied to the metal, the delocalised electrons are repelled by the negative terminal and attracted to the positive terminal.
The electrostatic attraction between metal ions in the lattice and delocalised electrons increases with an increasing number of electrons in d orbitals
As the number of valence electrons increases for a metal, the number of delocalised electrons moving throughout the lattice increases, increasing the overall electrical conductivity of the metal.
Explain the heat conductivity of transition metals
when a substance is heated it gains kinetic energy so their vibrations and movements becomes more vigorous.
When a metal is heated, the vibrations of its cations increase in magnitude and are passed along to other cations easily due to their closely packed nature
the vibrating cations also transfer energy to the surrounding electrons through collisions.
since the electrons in metals are mobile , they can pass increased kinetic energy to other parts of the lattice making them good conductors .
Explain thermal energy and resistence (not it kognity so notimprotant)
as thermal enegry increases ions in the lattice vibrate more so there are more collosions between electrons an ions
some kinetic energy is converted to ecah with every collosion
these collosions are the cause of electrical resistance in metals which increase with temperature
conversley, a decrease in temperature reduces the frequency of collosions, so the electrons move in a more direct path.
Why is copper harder than potassium
Because copper has both 4s and 3d electrons as delocalised valence electrons, there is an increased attraction between these electrons and the metal ions in the lattice. This increased strength of metallic bonds results in a greater hardness of the copper in comparison to potassium.