Chemistry - Atomic Structure and the Periodic Table
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
(a) Describe the postulates of Dalton’s theory (4)
(b) Describe the changes to Dalton’s theory (2)
(c) Identify, draw and label the three main different models of the atom (3)
(a) Describe the atom and its compositions (3)
(b) Describe the behaviour of subatomic particles in strong electric and magnetic fields (5)
(c) Draw and label the apparatus necessary to observe the behaviour of subatomic particles in a strong magnetic field
(a) Describe atomic number, atomic mass and relative atomic mass (3)
(b) Describe isotopes and how they are used to calculate the relative atomic mass of an atom (2)
(a) Describe radioactive isotopes
(b) Describe the types of emissions (3)
(c) Describe electron capture
(d) Describe the uses of five radioactive isotopes (5)
(a) Briefly describe Bohr’s theory on the orbit of electrons within the atom
(b) Describe emission spectra and explain how the phenomenon proves the existence of quantised energy levels
(c) Draw and label the hydrogen emission spectrum
(a) Describe quantum levels and their components (3)
(b) Describe the capacity of a sublevel to hold electrons and the order in which sublevels fill with electron (2)
(c) Describe the shape of s and p orbitals
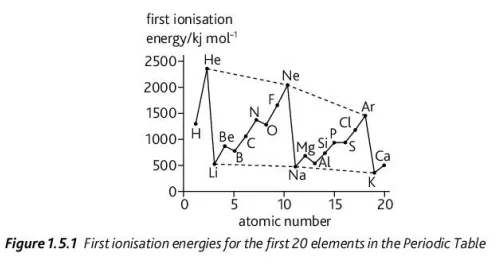
(a) Describe the ionization energy of an atom
(b) Differentiate between first and successive ionization energies
(c) Using the diagram, explain all trends in ionization energy (5)