chem unit 4 aos 1
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
88 Terms
organic compounds
carbon containing compounds formed by linking C tgt to make life possible
allotropes def
diff physical forms an element can exist in
why such a large diversity of organic compounds (why C can form so many compounds)
4 valence electrons → can form 4 strong covalent bonds
bonding can occur with diff element combos and in diff shapes
bond energy def
energy amount needed to break bonds a mole of molecules into ind atoms (intramolecular separation?)
kj mol^-1
indicates strength of covalent bond
bond energy depends on…
atoms involved in sharing covalent bond
distance between 2 atoms (bond length = distance between nuclei of the atoms sharing electrons) — shorter bonds usually more difficult to break
where is the evidence that carbon bonds are strong
the bond enthalpy table in databook showing large values
carbon bond angles
four single covalent bonds = tetrahedral distribution, carbon is saturated
double bond present (hence 2 remaining single bonds) = planar triangular distribution, unsaturated carbon (as one of the bonds in double bond weak → evidenced by bond enthalpy values in db)
triple bond (hence 1 remaining single bond) = linear distribution, unsaturated (2 of the bonds in triple bond weak)
how is shape of molecule determined
by max repulsion of electron pairs in molec (hence into diff distributions like tetrahedral, planar triangular, linear etc)
carbon bonds relative strength
between 2 C atoms:
triple bond > double bond > single bond
in terms of being shorter and stronger
how can degree of unsaturation be measured
reacting compound with iodine
the iodine number = mass iodine reacts with 100 g of comp
higher the number → the more iodine is reacted → the more unsaturated the comp is (the more double bonds there are? 1 mol of iodine reacts with 1 double bond?)
representing organic compounds
molecular formula
electron dot diagram (lewis struc)
structural formula
semi-struc (condensed) formula
skeletal formula
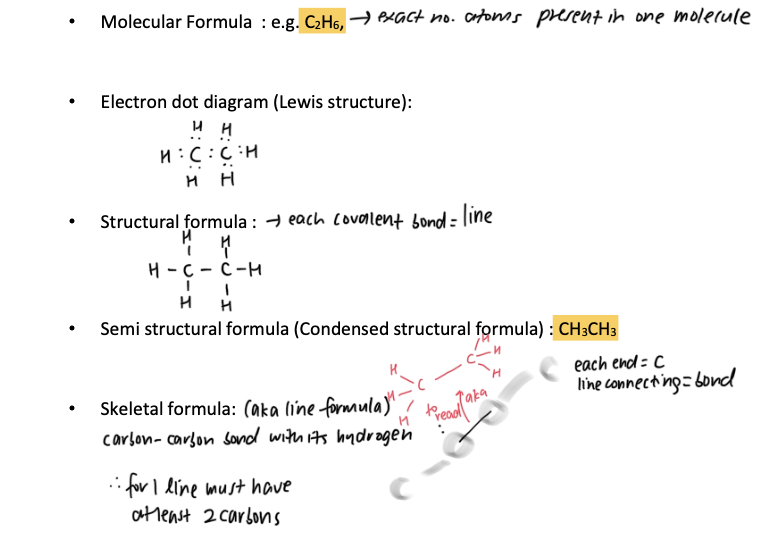
representing organic compounds — notes
semi-struc formula = written single line, each C atom followed by atoms joined to it, repeated CH2 gaps in brackets with subscript
skeletal = at each end of line (vertex) there is C atom (with enough H atoms bonded to it to satisfy C’s valency) — any other atoms or bonds apart from C — H shown on diagram normally
homologous series def
org compounds with:
similar strucs
similar chem properties
pattern to physical properties
same gen formula
— consecutive members of same homologous series differ by CH2
hydrocarbons def
simplest org compounds made only of C and H
— mainly gotten from crude oil
aliphatic vs aromatic compounds
aliphatic = org compounds where C atoms form open chains (alkanes, alkenes, alkynes)
aromatic = has one or more benzene rings, alternating single and double bonds within ring
alkanes
saturated hydrocarbons with only single covalent C — C or C — H bonds
gen formula = CnH2n+2
saturated def
all bonds strong covalent bonds + no multiple bonds between C atoms for one to be weaker hence allowing breakage (and addition of other atoms into molec)
alkenes
unsat hydrocarbons with at least 1 double bond between C atoms
gen formula = CnH2n
— (think as 2 hydrogen atoms removed from alkanes hence double bond must form — gen formula makes sense)
alkynes
unsat hydrocarbons with at least 1 triple bond between C atoms
gen formula = CnH2n-2
— not in sd?
cyclic hydrocarbons
hydrocarbon ring strucs where C chain is closed struc w/o open ends
(cycloalkane) gen formula = CnH2n ( same as alkenes !! - bc all C atoms covalently bonded to 2 others on either side)
diff molecular formula to straight-chain alkanes
prefix = cyclo- (e.g. cyclohexane)
arenes are a grp of this
benzene
produces arenes (aromatic, benzene-based hydrocarbons)
molec with 6 electrons from 3 double bonds shared by all the carbons in the ring — attraction of electrons to all C atoms gives molec stability
has alternating double and single bonds between C atoms in ring?
alkyl groups
hydrocarbon branches coming off longest C chain of org molecule
suffix = -yl
think as - if alkane has 1 or more H atoms removed (alkyl with same prefix as an alkane — the alkyl has 1 less H atom)
R
IUPAC naming org compounds
selection of main C chain
numbering of main C chain
naming (prefix + stem + suffix)
— see notes for more detail
functional grp def
atom/atoms attached to hydrocarbon chain that influences molec chem and physical properties
molec with functioning grp attached usually…
less stable than C backbone hence more likely participate chem reactions
homologous grp with functional grps — haloalkanes
one or more halogens attached to C chain
R — X (R = alkyl group/the hydroc chain, X = halogen)
prefix is halogen name with ‘ine’ ending replaced with ‘o’
CnH2n+1X
homologous grp with functional grps — amines
org compounds have amino functional grp
R — NH2
‘-amine’ suffix replaces ‘ane’ (but note that stem will still have ‘an’ attached like pentan-1-amine)
CnH2n+3N
homologous grp with functional grps — amides
org compounds with amide functional grp (-CONH-) where N attached to carbonyl C
R — CONH2
smallest primary amide has functional grp = -CONH2
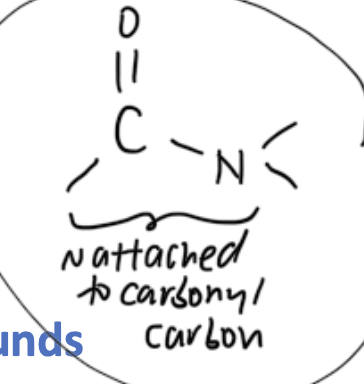
homologous grp with functional grps — alcohols
org compounds with hydroxyl (-OH) functional grp
R — OH
CnH2n+2O
primary alcohol (C-OH the alkyl C attached to one other C), secondary alcohol (C-OH the alkyl C attached to 2 other C), tertiary alcohol (C-OH the alkyl C attached to 3 other C)
carbonyl grp
when C double bonded to O
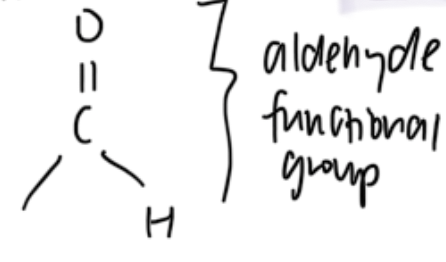
homologous grp with functional grps containing carbonyl grp — aldehydes
org compounds with aldehyde functional grp where H attached to carbonyl C
R — CHO
CnH2nO
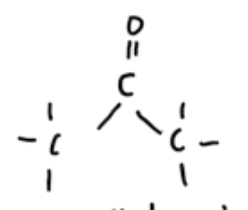
homologous grp with functional grps containing carbonyl grp — ketones
org compounds with ketone functional grp where 2 alkyl groups attached to carbonyl C
R — CO — R’
CnH2nO
homologous grp with functional grps containing carbonyl grp — carboxylic acids
org compounds with carboxyl functional grp (hydroxyl attached to carbonyl C)
R — COOH
CnH2nO2
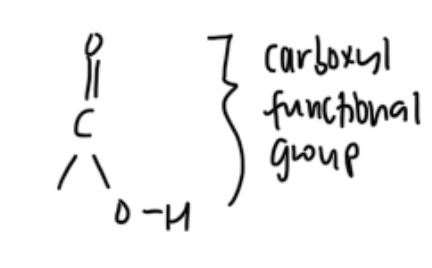
homologous grp with functional grps containing carbonyl grp — esters
org compounds with ester functional grp (O atom attached to carbonyl C and alkyl C)
R — COO — R’
the ester functional grp (-COO-) is called ester link → formed via condensation reaction between hydroxyl and carboxyl functional grps
name = stem of alcohol part + stem of main chain + oate (e.g. ethyl hexanoate) — note that the main chain must always include that carbonyl C
CnH2nO2
isomers def
comps same molecular formula but diff atom arrangements
(hence diff chem and physical properties)
structural isomers
comps same molecular formula but diff atom arrangement order
chain isomers
positional isomers
functional isomers
— diff IUPAC names
chain isomers
struc isomer type where diff atom arrangement order by changed main C chain
positional isomers
struc isomers type where diff atom arrangement order by changed position of functional grp (located on diff C atoms)
functional isomers
type struc isomer where diff atom arrangement order by changed functional grp
aldehyde and ketone
carboxylic acid and esters
alkene and cycloalkane
intermolecular forces
determines properties of substances
act between molecs
influenced by elements, bonds, shapes molecs
dispersion forces, dipole-dipole attractions, hydrogen bonding
intermolecular forces — dispersion forces
electrons momentarily distributed unevenly within molecs → temp dipole → neighbouring molecs with similar temp dipoles are attracted weakly with each other → weak dispersion forces between molecs
for non-polar molecs this is only intermolecular force → determines overall strength intermolecular bonding
weak and temporary (bc electrons redistribute themselves at diff times)
intermolecular forces — dipole-dipole attractions
molecs that polar and have permanent dipoles
partial +ve charge on one molec is electrostatically attracted to partial -ve charge on neighbouring molec
stronger than dispersion forces
intermolecular forces — Hydrogen bonding
when hydrogen bonds with F, N, O (highly electronegative atoms) its electrons move slightly towards atom → hence H nucleus exposed → the molec becomes dipole (+ve charge?) → H bonding occurs with this dipole and another molec with an electronegative atom
stronger than dipole-dipole attractions and dispersion forces (bc small size of H atom → larger dipole moment → molecs can get closer to each other → increased force of attraction
can occur between water and organic compounds
physical properties def
measurable + describes how subs behaves without changing chem composition
physical properties — boiling point and melting point
depends on strength of intermolecular forces (H bonding > dipole-dipole attractions > dispersion attractions)
as no. C atoms increase → increase dispersion forces → harder separate molecs → need higher temps
compounds with longer chain molecs → molecs can arrange closer → increased dispersion forces → increased intermolecular forces → harder separate molecs → higher temps
— the stronger the intermolecular forces, higher the boiling point
intermolecular forces for homologous grps
DISPERSION ONLY = alkanes, alkenes, alkynes
DISPERSION, DIPOLE-DIPOLE = haloalkanes, aldehydes, ketones, esters (HAKE)
DISPERSION, HYDROGEN BONDING = carboxylic acids, alcohols, amines, amides (CAAA)
physical properties — viscosity
resistance to flow of a liquid
larger molecs → increased dispersion forces → increased intermolecular forces → higher viscosity
temp increases → molecs have enough energy to overcome forces holding them tgt → viscosity decreases
— the stronger the intermolecular forces, higher viscosity
physical properties — solubility
for a subs to dissolve in water → molecs must interact with water → molecs separate so new interactions form
non-polar molecs cannot interact with water → can be attracted to non-polar solvents via dispersion forces
polar molecs → slightly soluble bc dipole-dipole attractions with water molecs → can separate so new interactions can form
molecs most likely dissolve in water are those can form H bonds
as org compounds molecs size increases → non-polar section of molec increases → solubility decreases
the effects of side-chains or branching on intermolecular force strength
branching amount increases → molecules cannot get as close to each other → as dispersion forces work for small distances, attraction reduced → bp and mp decrease
symmetrical branching increases → increased mp and bp
when explaining physical properties
use molec structure to justify type of intermolecular forces
explain how diff in intermolecular bonds results in diff properties
order of homologous grps intermolecular strengths (from highest to lowest)
carboxylic acid > amines / alcohols > esters / ketones / aldehydes / haloalkanes > hydrocarbons
substitution reactions def
one or more atoms in molec replaced by others
ORGANIC REACTIONS — alkane substitution
alkanes → strong covalent bonds and non-polar hence relatively unreactive
halogen can replace one or more H atoms
must happen under extreme conditions → UV light / heat — breaks covalent bond so reaction can occur
prod haloalkanes (primary haloalkanes = halogen attached to C atom that only attached one other C atom)
— e.g. halogens are Cl2, Br2 (fluorine too reactive, iodine too unreactive)
ORGANIC REACTIONS — haloalkane substitution
molecs polar bc electronegative halogens → can be subs with other atoms now (electron-rich grps)
if sub halog with -OH → prod alcohol
if sub halog with NH3 → prod amine
— note: if OH sub then e.g. can insert NaOH so that OH subs with the Cl in haloalkane and prod alcohol + NaCl as reaction products
addition reactions def
when one molec bonds covalently with another molec w/o losing atoms
ORGANIC REACTIONS — ALKENE ADDITION
unsat → weak bond in alkene double bond → break more easily → new single bonds form and new atoms added
— can occur with:
hydrogen (needs catalyst like Ni)
halogen
HCl
water (need H3PO4 catalyst at 300 degrees C) — note hence water is (g) state as steam
— addition polymerisation can also happen → alkene double bonds broken and each molec joined with others to form long chain (e.g. monomer = ethene, polymer = polyethene)
— note: when react alkene with Br2 can test for unsaturation as red-brown colour lost as bromine reacts
primary, secondary, tertiary alcohols
primary = C atom bonded to hydroxyl grp is only bonded to one other C atom (+ 2 other H atoms?)
secondary = C atom bonded to hydroxyl grp is bonded to 2 other alkyl grps / C atoms (+ one other H atom?)
tertiary = C atom bonded to hydroxyl grp bonded to 3 other alkyl grps / C atoms
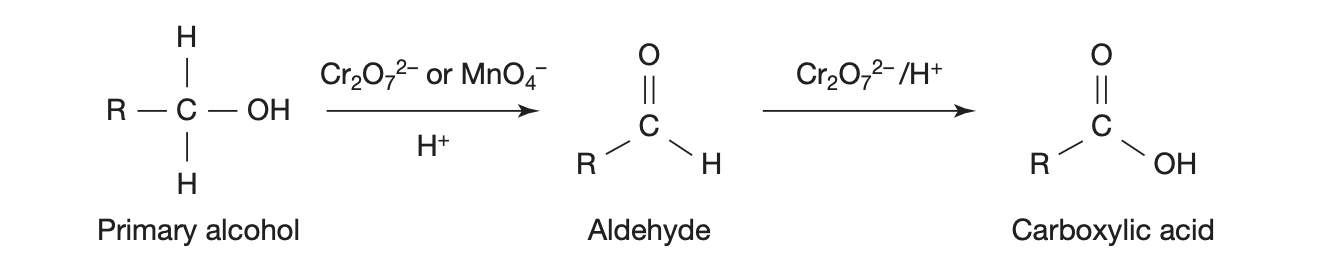
ORGANIC REACTIONS — primary alcohol oxidation
in presence of oxidant = MnO4-/H+ , Cr2O72-/H+
primary alcohol → (loses some H) → aldehyde → (O added?) → carboxylic acid
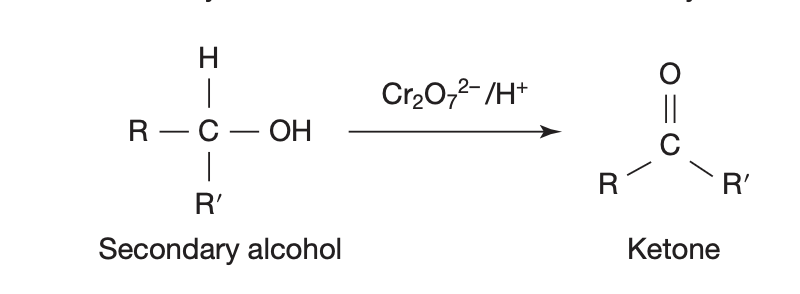
ORGANIC REACTIONS — secondary alcohol oxidation
in presence of oxidant = MnO4-/H+ , Cr2O72-/H+
secondary alcohol → (loses some H) → ketone
ORGANIC REACTIONS — tertiary alcohol oxidation
cannot be oxidised (bc can remove H atom easily from C hence cannot form double bond with O)
can separate primary and secondary alcohols from tertiary → tertiary cannot oxidise → hence Cr2O72- and MnO4- cannot cause colour change for tertiary but can for primary and secondary as they are reacting (orange to green, purple to pink respectively)
reaction pathway def
shows raw materials and sequence of steps to synthesise chem product
e.g. factors to choose a particular reaction pathway to manufacture chem products
cost and availability raw materials
energy cost
percentage yield
atom economy
etc
primary amine synthesis e.g. reaction pathways
alkane → haloalkane → amine
alkene → haloalkane → amine
alkene → alkane → haloalkane → amine
— note that need to conv into haloalkene first bc it’s more reactive → then heat with solution of concentrated ammonia in ethanol → amine mixture prod → separated via frac distillation
carboxylic acid synthesis e.g. reaction pathways (can also then prod ester)
alkane → haloalkane → alcohol → carboxylic acid (→ ester)
note: the further down the grp the halogen is — the faster OH replaces it → faster reaction rate
AH ACE
condensation reaction def
smaller molecs combine to form bigger molec by forming covalent bonds and lose small molec like H2O
hydrolytic reaction def
breakdown of larger molec into smaller molecs bc addition of water to break covalent bonds
ORGANIC REACTIONS — carboxylic acid condensation
carboxylic acid + alcohol → ester + water
concentrated acid catalyst (H+ above arrow) — e.g. sulfuric acid H₂SO₄ (l)
OH leaves from carboxylic acid and H leaves from alcohol hydroxyl grp → forms bond between the O to join molecs
also need heat?
ORGANIC REACTIONS — ester hydrolysis
ester + water → carboxylic acid + alcohol
if (dilute) acid catalyst then reversible, if alkaline catalyst (hydroxide ions) then salt and alcohol forms instead hence reaction is one-way — like H2SO4 (aq) vs KOH (s)
need heat? also just use catalyst as H2SO4 (l)?
biodiesel def
diesel alternative fuel prod from plant oils/animal fats and alcohol
triglycerides
naturally occurring ester formed from condensation of 3 fatty acids and glycerol
fatty acids = 12-20 C atoms carboxylic acids
glycerol loses H+ (from OH side) and each fatty acid loses OH- then bond between the gap to form triglyceride (bond between C from COOH fatty acid and O from glycerol) → prod water
have large non-polar sections → hence hydrophobic
3 fatty acids + glycerol → triglyceride + 3 H2O
biodiesel production
— transesterification reaction:
triglyceride + 3 methanol → 3 biodiesel molecules + glycerol (and catalyst above arrow)
triglyceride — glycerol part to glycerol → rest to biodiesel molecule
methanol — CH3OH → H+ to complete glycerol → CH3O- to biodiesel molecule
heat and conc NaOH or conc KOH (catalyst) used
— several small alcohols can be used → if methanol used → prod methyl ester
(note the biodiesel molec prod from a fatty acid is just that fatty acid semi-struc formula from data book but with COOH replaced with COOH3C instead?)
obtaining methanol
— non-renewable fossil fuels:
steam reforming to prod synthesis gas → further reactions to make methanol
natural gas (methane) as feedstock: CH4 (g) + H2O (g) → CH3OH (g) + H2 (g)
— renewable resources
glycerol (from the transesterification reaction) as feedstock → prod synthesis gas → further reactions to make methanol
use catalyst for direct conversion glycerol → methanol
esterification def
condensation reactions that result in ester formation
transesterification def
converting one ester into another
metabolism def
chem processes within living org to maintain life (e.g. nutrient digestion, breakdown of prots carbs fats and oils)
enzymes
prot acts as biological catalyst
needed for specific hydrolysis and condensation reactions
only function effectively at specific temps and pH — depend on body area
proteins
polypeptides (condensation polymers) made from amino acid monomers
broken down into amino acids via hydrolysis → water added → breaks peptide link (CONH) between amino acids → forms NH2 on one amino acid and COOH on the other
amino acids used by body to form prots it needs → condensation reaction between amino acids so polymer forms via peptide links → NH2 loses H and COOH loses OH from each amino acid, peptide link between → the remains of each amino acid in peptide (there is N-terminal end and C-terminal end) called residue while water prod
draw open bonds at ends when drawing polymer segment
peptide chain of C and N atoms is backbone, R grps are side chains
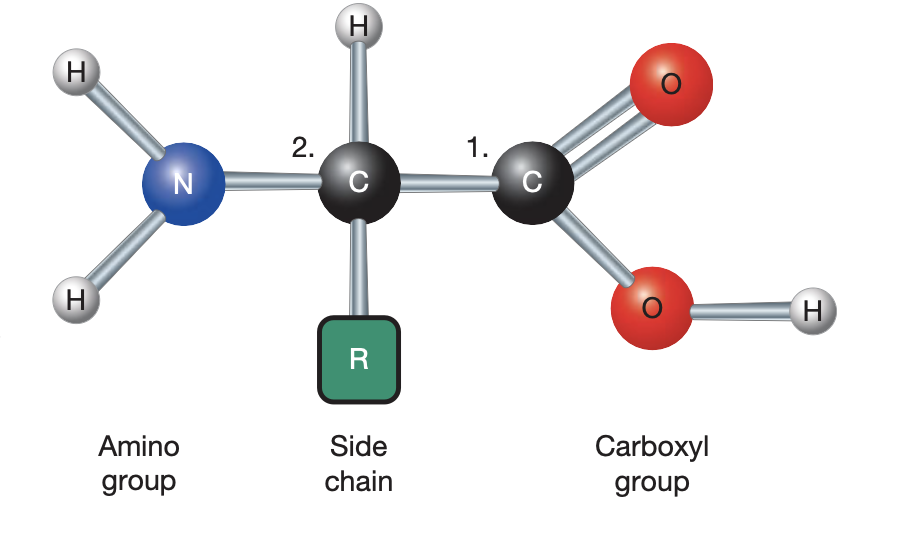
amino acids
have amino grp, carboxyl grp, R grp, H atom
20 diff amino acids in human prots — all diff R grps (side chains)
protein monomers
carbohydrates
Cx(H2O)y — ratio of H:O in carbs always 2:1
can be monosaccharides, disacch, polysacch
most from plants as glucose via photosynthesis
glucose is readily available energy source
polysacch formed from condensation polymerisation between monosacchs / disacchs via glycosidic link
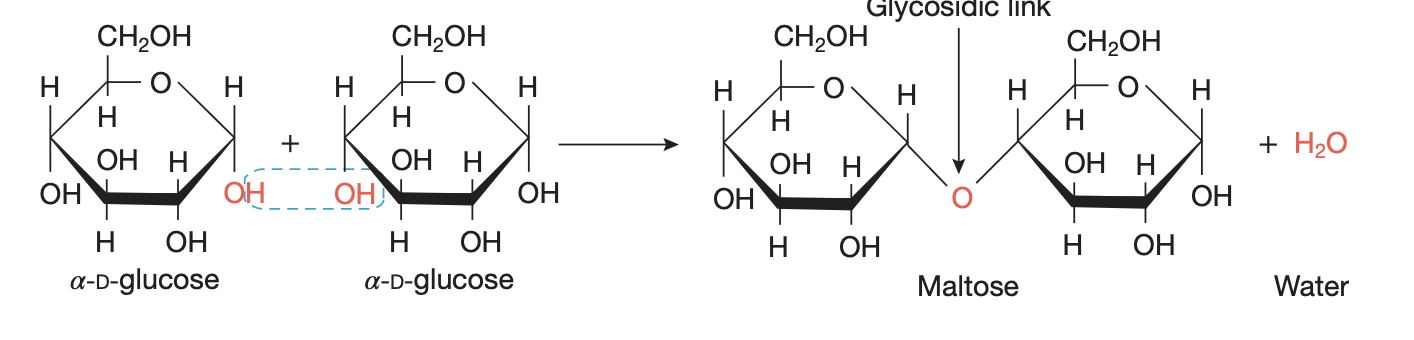
carb types
monosaccharide = glucose in simplest form, soluble hence not storable
disaccharide = 2 glucose molecs bonded via glycosidic bond from condensation reaction, soluble hence not storable
polysaccharide = more than 10 glucose molecs bonded tgt, insoluble hence storable (starch, glycogen, cellulose)
carbs — polysaccharides
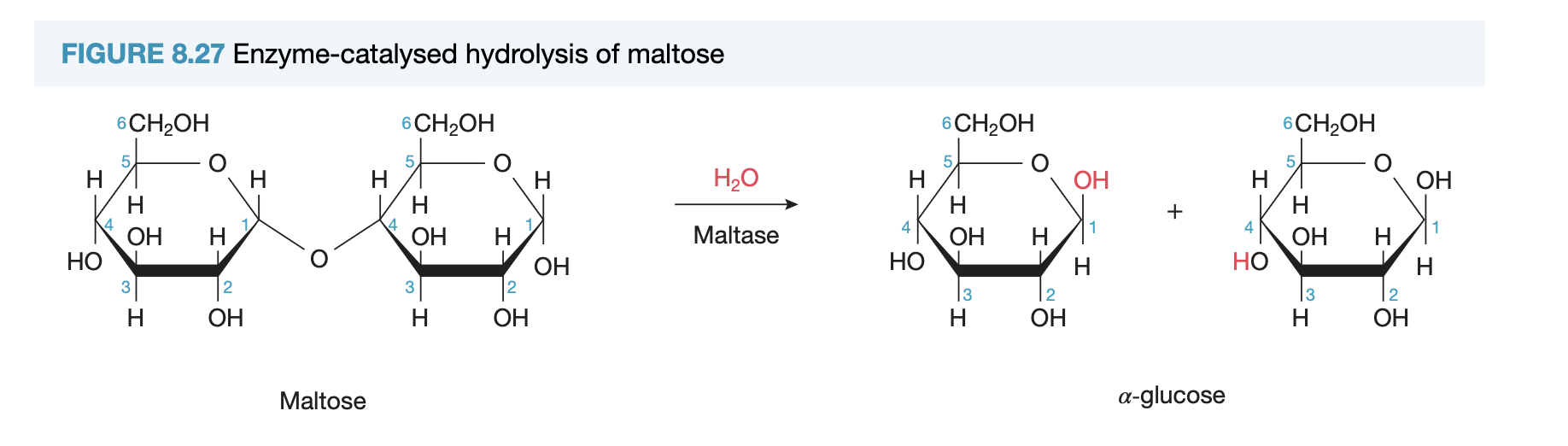
starch: hydrolysed in mouth by amylase (enzyme) to prod maltose (disacch) by added water breaking glycosidic links → then further hydrolysis to monosacch at intestine, by breaking glycosidic links between disacch pairs
— formed via condensation of glucose → join H atom on one monomer and OH on another to eliminate water
glycogen: stores glucose when not needed in liver, more highly branched + shorter than starch, liver reconverts glycogen → glucose via hydrolysis
— formed via condensation polymerisation of glucose
fats and oils (triglycerides)
type of lipids
not polymers
hydrophobic, less dense than water
digestion in intestine with bile as emulsifier → after then hydrolysis → added water breaks triglyceride to form one glycerol, 3 fatty acids (H to glycerol, OH to fatty acids)
formed via condensation between 3 fatty acids and 1 glycerol → H from glycerol and OH from fatty acids leave to form 3 water → ester link forms between to form trigly.
percentage yield as a measure of chemical process efficiency
% yield = actual yield / theoretical yield x 100
theoretical yield from amount of product expected from stoic ratio of limiting reagent
why actual yield lower than theoretical = equilibrium reached, waste, side reactions (due to slower target reaction rate hence other products formed instead)
to calc % yield for multi-step pathways multiple yields of each step
green chemistry
need to change from secondary prevention (costly cleaning of wastes after prod) to primary prevention (development manufacturing processes not polluting)
principles in datab.
atom economy
a measure of how many reactant atoms end up in desired product
aim to maximise reactant atoms for final product and decrease amount waste prod
% atom economy = molar mass of desired product / molar mass all reactants x 100
for two-step reaction the intermediate products are removed from calcs
using renewable feedstocks
raw materials can be replenished in less time than consumed
e.g. biomass
need new tech and more research to implement in society more
choosing catalysts
when increase reaction rate can allow processes carried out milder conds (temp, pressure) → saves energy
only small amount catalyst needed and not consumed hence sustainable usage
but some catalysts not green like heavy metals (depleting, hazard to health)
heterogenous catalysts pref bc easier separate from products → reduce no. steps in process
microorgs as biocatalysts to accelerate reactions → need milder conds, prod less waste, less hazardous, use less steps, saves energy compared to synthetic catalysts → but also need specific conds which may be hard to create (temp, pH, etc)
highly specific → can be designed for specific reactions for more pathway control → reduces side reactions
designing safer chemicals
chems should be designed with minimal toxicity w/o reducing effectiveness
removing hazardous subs needed for workers health + prevent envo damage