Biochemistry online notecards
1/44
Earn XP
Description and Tags
so I can study and get extra credit
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
How to find number of protons and electrons
its equal to the atomic number
number of neutrons
the rounded atomic mass and subtract protons
What will happen if you changed the number of neutrons
an isotope and change in mass
if you change number of protons
a charged ion (+/-)
How much electrons go on each shell
1st shell- 2
after 1st shell-8
What if you change number of electrons
A charged ion (positive or negative)
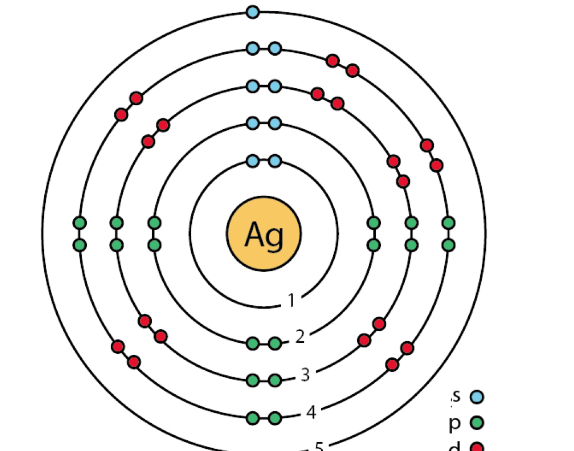
What is this?
An example of a Bohr model
The octet rule
Atoms gain lose or share their electrons until their valence shell is full
Covalent bond
Sharing pair(s) of electrons between the atoms
Ionic bond
Results from the transfer of electrons from a metal to a non metal
What does the C stand for in CHNOPS
Carbon
What does the H stand for in CHNOPS
Hydrogen
What does the N stand for in CHNOPS
Nitrogen
What does the O stand for in CHNOPS
Oxygen
What does the P stand for in CHNOPS
Phosphorus
What does the S stand for in CHNOPS
Sulfur
One atom holds the shared electron for a longer time
Polar
Atoms share electrons equally
nonpolar
The dotted lines indicate hydrogen bonds and the solid lines indicate polar covalent bonds
.
Hydrogen bond
Attraction between the partial charges in a hydroge and a partial negative charge
Adhesion
Water sticks to other substances
Transpiration
Evaporation of water from the leaves of a tree
Why is floating ice important
Oceans and lakes don’t freeze solid
Specific heat
Amount of heat needed to raise or lower 1g of a substance 1 degree celcius
Heat of vaporization
Amount of energy to convert 1g of a substance from a liquid to a gas
In order for water to evaporate ALL hydrogen bonds must be broken
…
How many bonds can carbon make?
4
When carbon makes bonds what happens
shares electrons with other atoms
Carbons are shown as a point (corner) and the end of lines. H is not shown at all.